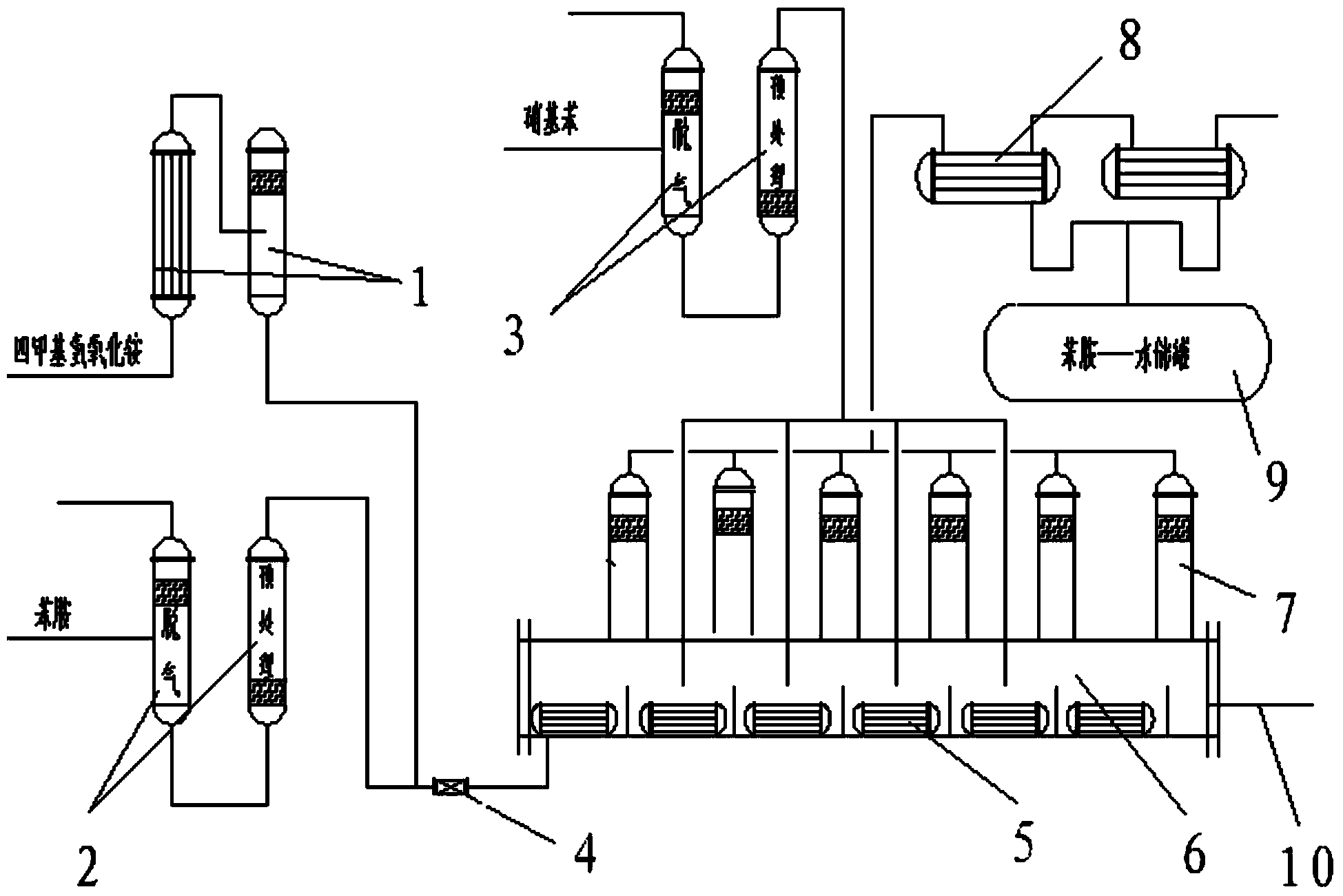
Production equipment and production technology of precursor of 4-aminodiphenylamine
A technology of aminodiphenylamine and production process, applied in the direction of condensation/addition reaction to prepare amino compounds, organic chemistry, etc., can solve the problems of easy decomposition and recovery, difficulty, etc., and achieve strong continuity, low environmental pollution, and high degree of automation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0022] Embodiment (industrialized device embodiment)
[0023] First, turn on the nitrobenzene, aniline, and quaternary ammonium tetramethylammonium hydroxide catalyst delivery pumps to control the flow rate of aniline to 13.2 cubic meters per hour and the flow rate of nitrobenzene to 2 cubic meters per hour through flow adjustment, respectively passing through the catalysts filled with alkali metal hydrogen Vacuum degassing washing tank treatment of oxides (30%NaOH), the purpose is to remove the acidic substances dissolved and adsorbed in the raw materials and some gases that are not conducive to the condensation reaction of nitrobenzene and aniline, after alkali metal hydroxide treatment and degassing The final nitrobenzene (added at 4 points in different positions of the tubular condensation reactor) is 0.6 cubic per hour, 0.6 cubic per hour, 0.5 cubic per hour, and 0.3 cubic per hour from the starting point, and passes through the alkali metal hydroxide After treatment and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com