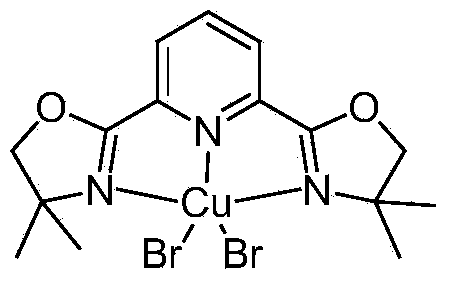
Copper bromide reagent, preparation method thereof, and synthesis method of alpha-amido propiophenone and derivative thereof
A synthesis method, copper bromide technology, applied in chemical instruments and methods, copper organic compounds, chemical/physical processes, etc., can solve the problem of unsuitable large-scale industrial production, expensive raw materials of azo compounds, long reaction time, etc. problem, to achieve the effects of good reactivity, green synthesis route, and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] In a 50ml one-necked flask, add a polytetrafluoroethylene magnetic stirrer, 0.2mmol copper bromide, 0.20mmol 2,6-bis[4-(1,1-dimethyl)-2-oxazoline-2- Base] pyridine, 15ml methanol and 10ml dichloromethane solvent; after ultrasonic mixing, react at 20°C under nitrogen protection for 5 hours. The obtained mixture was removed with a rotary evaporator to remove the solvent, and washed with methanol and ether to obtain a light green solid, which was washed with ether and dried to obtain 95 mg (96%) of a light green solid, which was 2,6-bis[4-( 1,1-Dimethyl)-2-oxazolin-2-yl]pyridinated copper bromide coordination compound, namely copper bromide reagent.
Embodiment 2
[0022] In a 50ml one-necked flask, add a polytetrafluoroethylene magnetic stirrer, 0.1mmol copper bromide, 0.5mmol 2,6-bis[4-(1,1-dimethyl)-2-oxazoline-2- Base] pyridine, 15ml methanol and 10ml dichloromethane solvent; after ultrasonic mixing, react at 15°C under nitrogen protection for 5 hours. The obtained mixture was removed with a rotary evaporator to remove the solvent, and washed with methanol and ether to obtain a light green solid, which was washed with ether and dried to obtain 48 mg (96%) of a light green solid, which was 2,6-bis[4-( 1,1-Dimethyl)-2-oxazolin-2-yl]pyridinated copper bromide coordination compound, namely copper bromide reagent.
Embodiment 3
[0024] In a 50ml one-necked flask, add a polytetrafluoroethylene magnetic stirrer, 0.5mmol copper bromide, 0.1mmol 2,6-bis[4-(1,1-dimethyl)-2-oxazoline-2- Base] pyridine, 15ml methanol and 10ml dichloromethane solvent; after ultrasonic mixing, react at 23°C under nitrogen protection for 6 hours. The resulting mixture was removed from the solvent with a rotary evaporator, washed with methanol and ether to obtain a light green solid, washed with ether, and dried to obtain 48 mg (96%) of a light green solid, which was 2,6-bis[4-(1 ,1-Dimethyl)-2-oxazolin-2-yl]pyridinated copper bromide coordination compound, that is, copper bromide reagent.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com