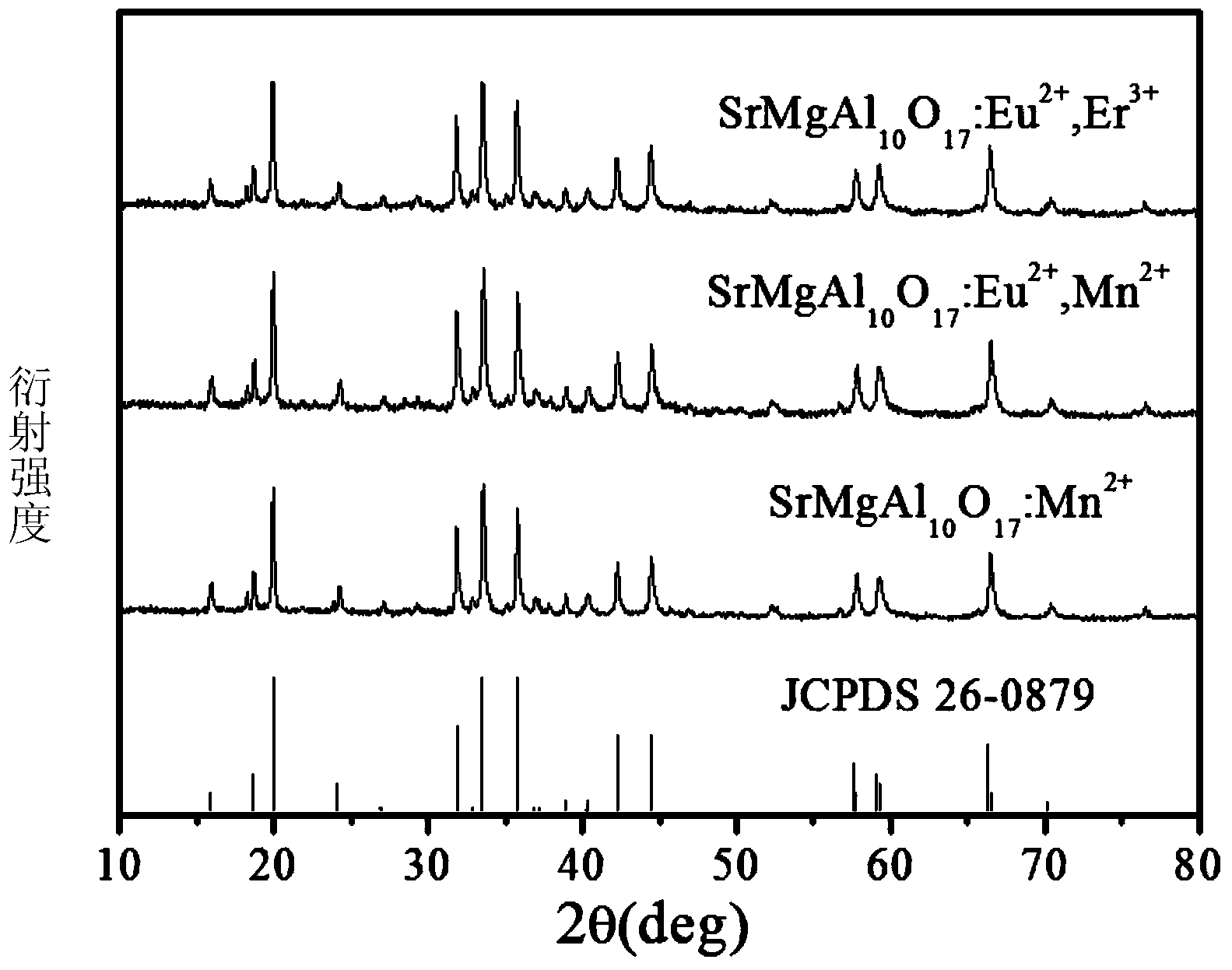
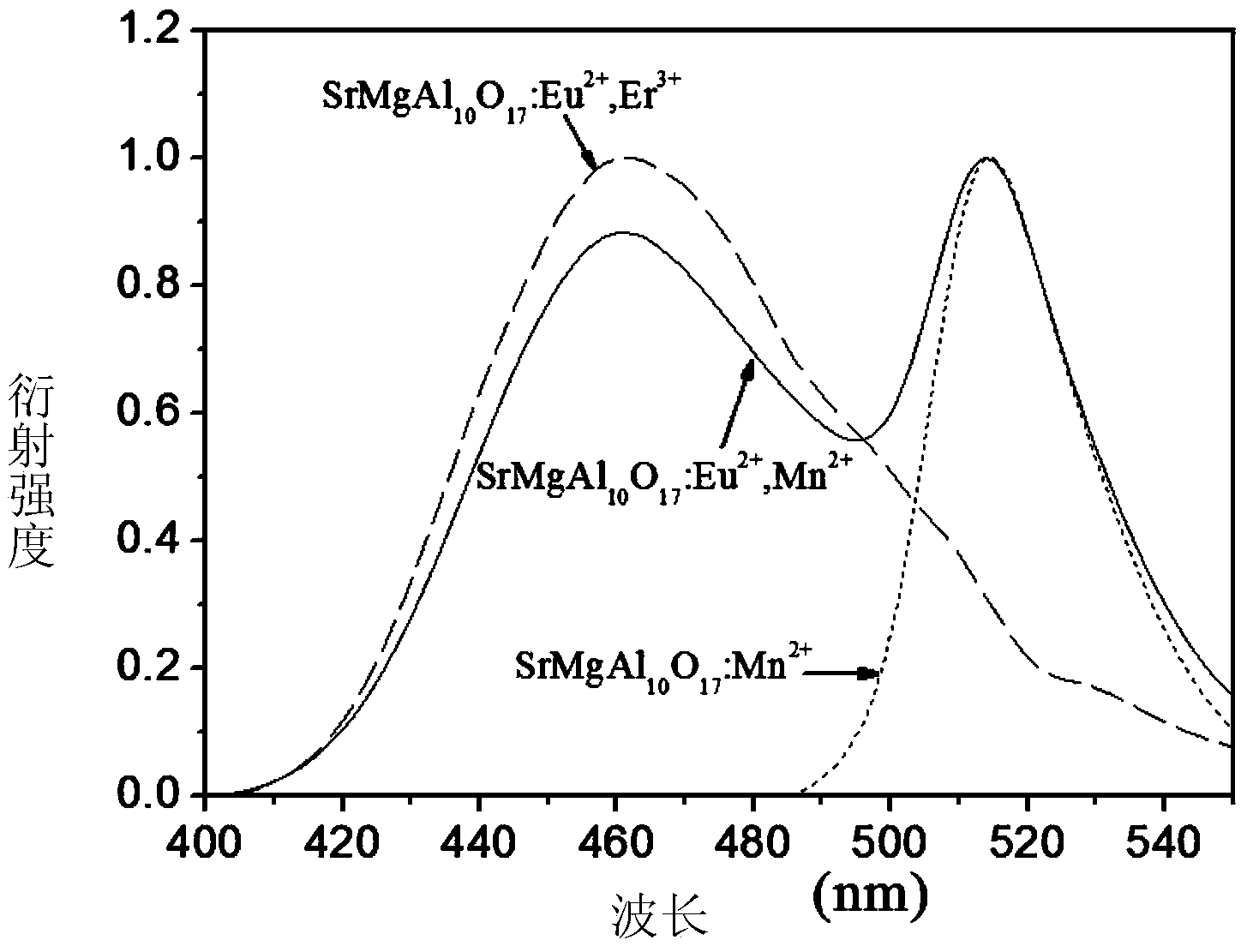
Method for preparing blue aluminate fluorescence powder via sol self-combustion method
A technology of blue fluorescent powder and self-combustion method, which is applied in the direction of chemical instruments and methods, luminescent materials, etc., can solve the problems of complex process, time-consuming and energy consumption, etc., and achieve the effect of simple process, uniform distribution and low synthesis temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Accurately weigh 7.5027g Al(NO 3 ) 3 9H 2 O, 0.3852g Sr(NO 3 ) 2 , 0.5128gMg (NO 3 ) 2 ·6H 2 O, 0.0320g Eu 2 o 3 and 0.0115g MnCO 3 . Then press nitrate [Al(NO 3 ) 3 9H 2 O, Sr(NO 3 ) 2 and Mg(NO 3 ) 2 ·6H 2 The amount of the total substance of the three]: urea is the urea added by weighing the molar ratio of 1:1.5, according to nitrate [Al(NO 3 ) 3 9H 2 O, Sr(NO 3 ) 2 and Mg(NO 3 ) 2 ·6H 2 The amount of the total substance of the three]: boric acid is the boric acid added by weighing the mol ratio of 100:1, by every mole of Al (NO 3 ) 3 9H 2 Add concentrated nitric acid in the amount of 50ml of O, that is, Al(NO 3 ) 3 9H 2 O is 0.02 mol, and the nitric acid in the added concentrated nitric acid is 0.015 mol, according to each mole of Al(NO 3 ) 3 9H 2 O is the amount that 150ml adds dehydrated alcohol, namely Al(NO 3 ) 3 9H 2 O is 0.02mol, and the absolute ethanol added is 0.05138mol.
[0030] Will Eu 2 o 3 , MnCO 3 Dissolve in co...
Embodiment 2
[0032] Accurately weigh 7.5027g Al(NO 3 ) 3 9H 2 O, 0.3852gSr(NO 3 ) 2 , 0.5128gMg (NO 3 ) 2 ·6H 2 O, 0.0320g Eu 2 o 3 and 0.0161g MnCO 3 ; Press nitrate [Al(NO 3 ) 3 9H 2 O, Sr(NO 3 ) 2 and Mg(NO 3 ) 2 ·6H 2 The amount of the total substance of the three]: urea is the urea added by weighing the molar ratio of 1:1.5, according to nitrate [Al(NO 3 ) 3 9H 2 O, Sr(NO 3 ) 2 and Mg(NO 3 ) 2 ·6H 2 The amount of the total substance of the three]: boric acid is the boric acid added by weighing the mol ratio of 100:1, by every mole of Al (NO 3 ) 3 9H 2 Add concentrated nitric acid in the amount of 50ml of O, that is, Al(NO 3 ) 3 9H 2 O is 0.02 mol, and the nitric acid in the added concentrated nitric acid is 0.015 mol, according to each mole of Al(NO 3 ) 3 9H 2 O is the amount that 150ml adds dehydrated alcohol, namely Al(NO 3 ) 3 9H 2 O is 0.02mol, and the absolute ethanol added is 0.05138mol.
[0033] Will Eu 2 o 3 , MnCO 3 Dissolve in concentra...
Embodiment 3
[0035] Accurately weigh 7.5027g Al(NO 3 ) 3 9H 2 O, 0.42326g Sr(NO 3 ) 2 , 0.5128gMg (NO 3 ) 2 ·6H 2 O, 0.0115g MnCO 3 ; Press nitrate [Al(NO 3 ) 3 9H 2 O, Sr(NO 3 ) 2 and Mg(NO 3 ) 2 ·6H 2The amount of the total substance of the three]: urea is the urea added by weighing the molar ratio of 1:1.5, according to nitrate [Al(NO 3 ) 3 9H 2 O, Sr(NO 3 ) 2 and Mg(NO 3 ) 2 ·6H 2 The amount of the total substance of the three]: boric acid is the boric acid added by weighing the mol ratio of 100:1, by every mole of Al (NO 3 ) 3 9H 2 Add concentrated nitric acid in the amount of 50ml of O, that is, Al(NO 3 ) 3 9H 2 O is 0.02 mol, and the nitric acid in the added concentrated nitric acid is 0.015 mol, according to each mole of Al(NO 3 ) 3 9H 2 O is the amount that 150ml adds dehydrated alcohol, namely Al(NO 3 ) 3 9H 2 O is 0.02mol, and the absolute ethanol added is 0.05138mol.
[0036] MnCO 3 Dissolve in concentrated nitric acid to obtain a transparent ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com