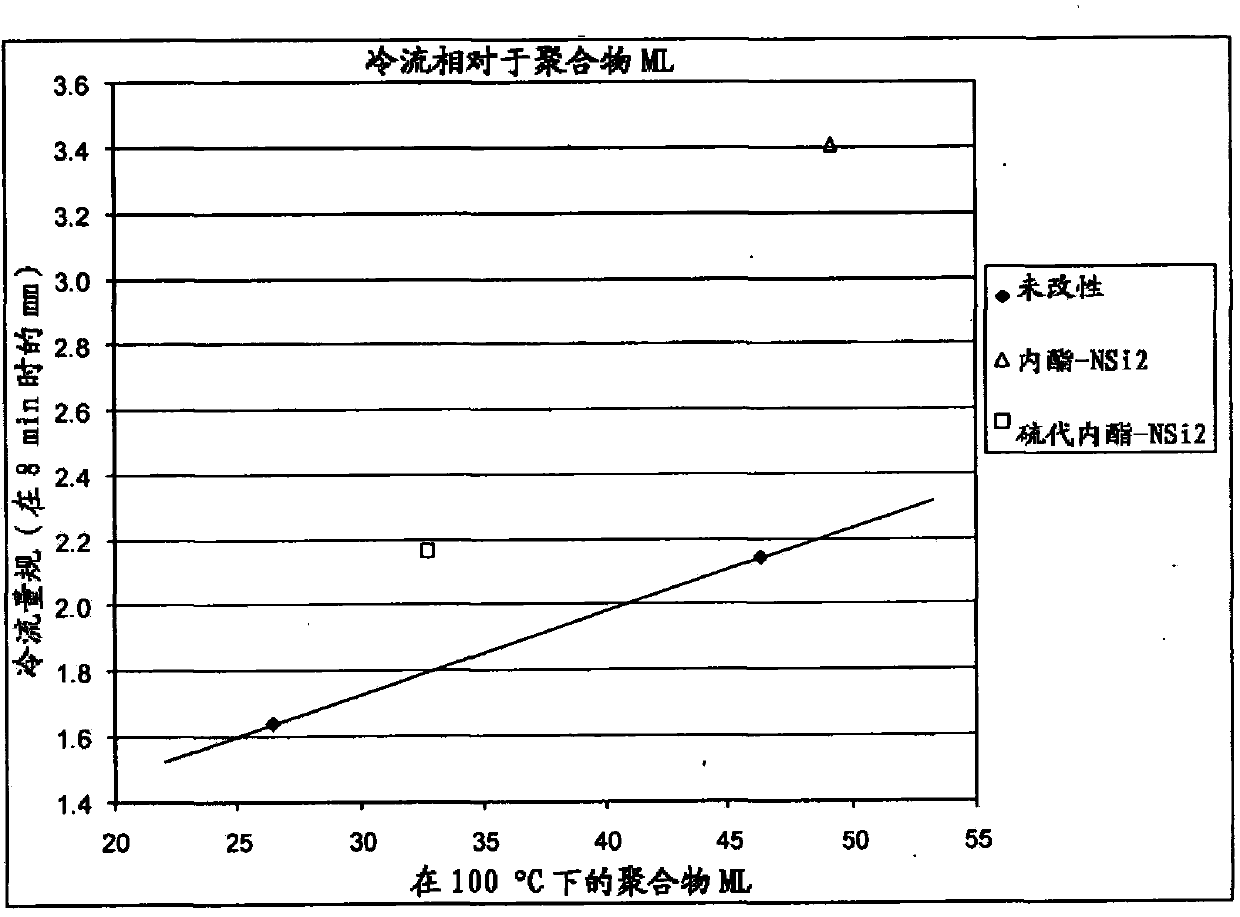
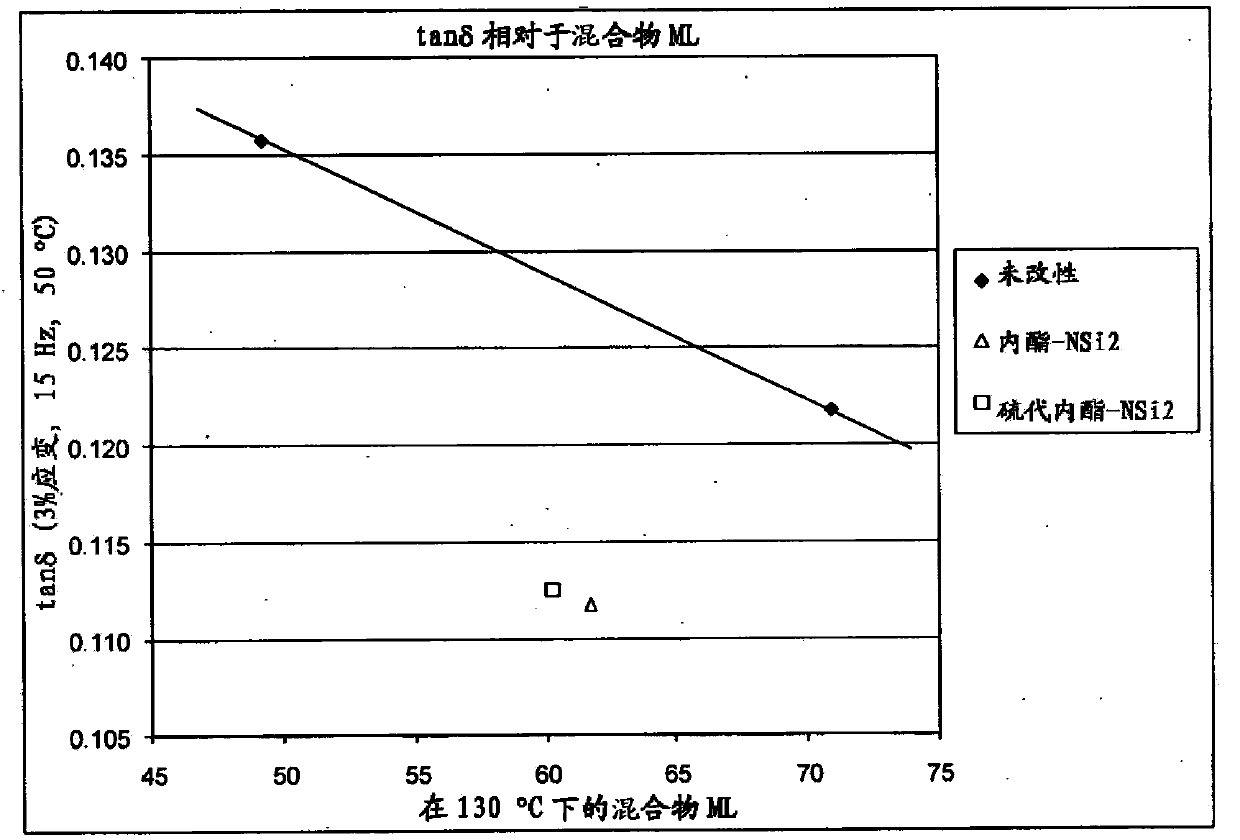
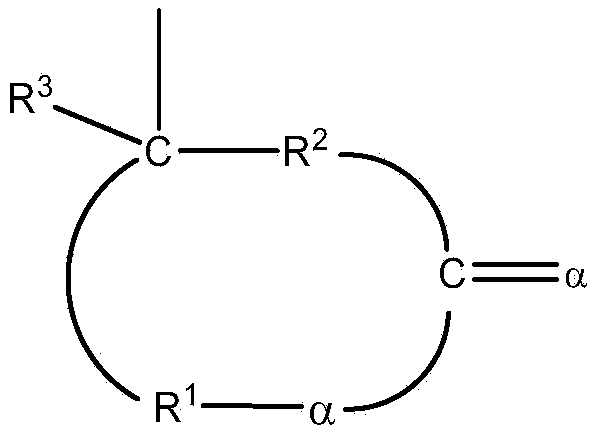
Polymers functionalized with lactones or thiolactones containing a protected amino group
A thiolactone, polymer technology, used in transportation and packaging, special tires, tire parts, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0097] The preparation of reactive polymers according to the invention can be achieved by polymerizing conjugated diolefins, optionally together with monomers copolymerizable with conjugated diene monomers, in the presence of a catalytically effective amount of a catalyst or initiator. vinyl monomers. The introduction of catalyst or initiator, conjugated diene monomer, optional comonomer, and any solvent (if used) forms the polymerization mixture in which the reactive polymer is formed. The amount of catalyst or initiator to be used can depend on the interplay of various factors, such as the type of catalyst or initiator used, the purity of the ingredients, the polymerization temperature, the desired rate and conversion of polymerization, the desired molecular weight and many other factors. Accordingly, no specific amount of catalyst or initiator can be definitively described other than to indicate that a catalytically effective amount of catalyst or initiator may be used.
...
example 1
[0212] Example 1. α-(2,2,5,5-tetramethyl-1-aza-2,5-disila-1-cyclopentyl)-γ-butyrolactone (lactone-NSi 2 )Synthesis
[0213] α-Amino-γ-butyrolactone hydrobromide (9.01 g, 0.050 mmol) and dichloromethane (30 mL) were mixed in an ice-bath cooled round bottom flask. To this mixture was added triethylamine (16.70 g, 0.165 mmol), and a solution of 1,2-bis(chlorodimethylsilyl)ethane (10.76 g, 0.050 mmol) in dichloromethane (50 ml) . The resulting mixture was stirred at room temperature for 47 hours, then evaporated under vacuum. The residue was extracted with 500 ml cyclohexane and filtered through frit. The filtrate was evaporated under vacuum to yield α-(2,2,5,5-tetramethyl-1-aza-2,5-disila-1-cyclopentyl)-γ-butyrolene as a white solid Esters (abbreviated as Lactone-NSi 2 ) (11.8 g, 97% yield). product of 1 H NMR data (C 6 D. 6 , 25°C, referring to tetramethylsilane) as follows: δ3.50 (multiplet, 1H), 3.33 (multiplet, 1H), 3.22 (multiplet, 1H), 1.64 (multiplet, 1H), 1.50 ( ...
example 2
[0215] Example 2. α-(2,2,5,5-tetramethyl-1-aza-2,5-disila-1-cyclopentyl)-γ-thiobutyrolactone (thiolactone -NSi 2 )Synthesis
[0216] DL-homocysteine thiolactone hydrochloride (7.68 g, 0.050 mmol) and dichloromethane (30 mL) were mixed in a round bottom flask cooled with an ice bath. To this mixture was added triethylamine (16.70 g, 0.165 mmol), and a solution of 1,2-bis(chlorodimethylsilyl)ethane (10.76 g, 0.050 mmol) in 50 ml of dichloromethane. The resulting mixture was stirred at room temperature for 47 hours, then evaporated under vacuum. The residue was extracted with 500 ml cyclohexane and filtered through fritted glass. The filtrate was evaporated under vacuum to yield α-(2,2,5,5-tetramethyl-1-aza-2,5-disila-1-cyclopentyl)-γ-thioxo as a white solid Butyrolactone (abbreviated as Thiolactone-NSi 2 ) (11.8 g, 97% yield). product of 1 H NMR data (C 6 D. 6 , 25°C, referring to tetramethylsilane) are listed as follows: δ3.24 (multiplet, 1H), 2.34 (multiplet, 1H), 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com