Medicine for treating puerperal fever
A postpartum fever and drug technology, applied in the field of medicine, can solve problems such as poor fever effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example
[0017] Diagnostic criteria
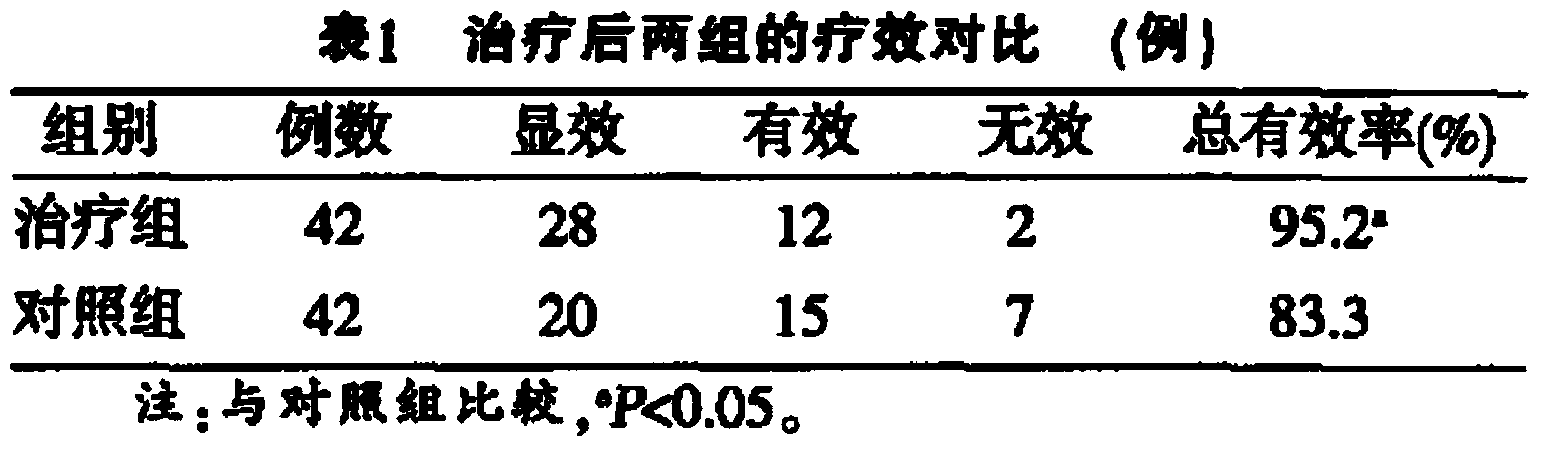
[0018] According to (Tian Bing. Observation on clinical efficacy of Jiawei Shenghua Decoction in treating postpartum fever [J]. Chinese Community Physician (Medical Profession), 2011, 12(33): 149-150) to formulate diagnostic criteria: persistent fever within 10 days after delivery, or The body temperature above 38°C lasted for 3 days, accompanied by abdominal pain and abnormal changes in the color, quality, quantity, and smell of vaginal secretions. The pathogenic bacteria and the site of infection were identified through routine blood and urine examinations and culture of uterine secretions.
[0019] normal information
[0020] A total of 84 patients with postpartum fever admitted to the Obstetrics and Gynecology Department of our hospital from May 2012 to November 2013 were selected and divided into a treatment group and a control group, with 42 cases in each group. The average age of the treatment group was (24.6±3.8) years old; 26 primiparous ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com