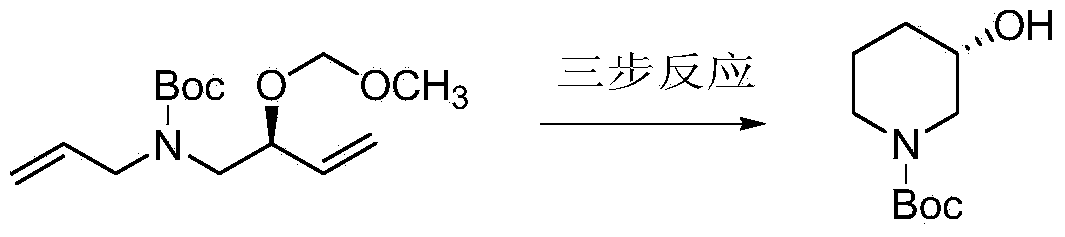
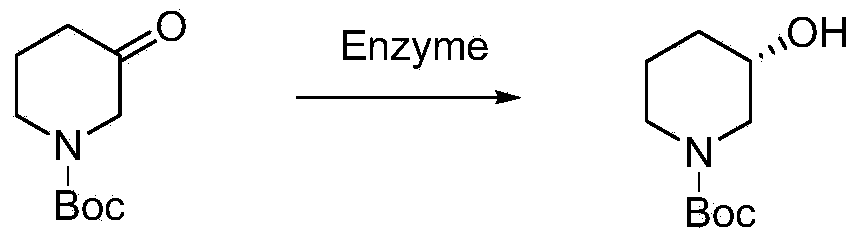
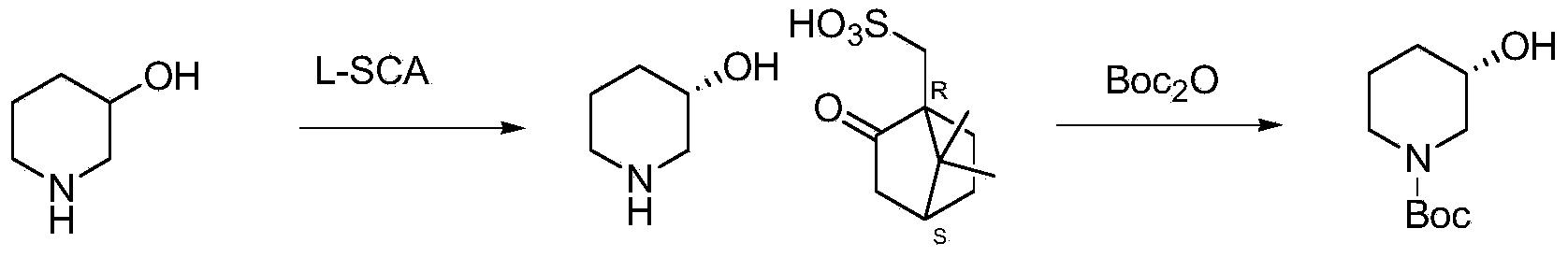
Preparation of chirality-1-t-butyloxycarboryl-3-hydroxy piperidine and method for chirality turning
A technology of tert-butoxycarbonyl and hydroxypiperidine, which is applied in the field of compound synthesis, can solve the problems of difficult industrialization, chiral impurities in the resolution process, and difficult purification, etc., and achieve the effect of clean reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: the synthesis of (S)-1-benzyl-3-hydroxypiperidine camphorsulfonate
[0040] (1) Synthesis of (S)-1-benzyl-3-hydroxypiperidine camphorsulfonate (1)
[0041] In a 1000ml three-necked flask, add N-benzyl-3-hydroxypiperidine (95.6g, 0.5mol), add 478ml of isopropanol dropwise in 116ml of isopropanol solution of L-CSA (58g, 0.25mol), and stir at room temperature for 1 hour , a solid precipitated out, kept at 0°C for 2 hours, filtered, washed with 30 ml of cold isopropanol, and dried to obtain 75 g of (S)-1-benzyl-3-hydroxypiperidine camphorsulfonate. (ee: 95%) (theoretical: 105.9g). (S)-1-benzyl-3-hydroxypiperidine camphorsulfonate with 95% ee value is recrystallized with 3 times the amount of isopropanol to obtain (S)-1-benzyl-3-hydroxypiperidine Camphor Sulfonate (99% ee).
[0042] (2) Synthesis of (S)-1-benzyl-3-hydroxypiperidine camphorsulfonate (2)
[0043] In a 1000ml three-necked flask, add N-benzyl-3-hydroxypiperidine (95.6g, 0.5mol), add 478ml of et...
Embodiment 2
[0052] Embodiment 2: the synthesis of (R)-1-benzyl-3-hydroxypiperidine camphorsulfonate
[0053] In a 1000ml three-necked flask, add N-benzyl-3-hydroxypiperidine (95.6g, 0.5mol), add D-CSA (58g, 0.25mol) in 116ml of isopropanol solution in 478ml of isopropanol, and stir at room temperature for 1 hour , a solid precipitated out, kept at 0°C for 2 hours, filtered, washed with 30 ml of cold isopropanol, and dried to obtain 75 g of (R)-1-benzyl-3-hydroxypiperidine camphorsulfonate. (ee: 95%) (theoretical: 105.9g). (R)-1-benzyl-3-hydroxypiperidine camphorsulfonate with 95% ee value is recrystallized with 3 times the amount of isopropanol to obtain (R)-1-benzyl-3-hydroxypiperidine Camphor Sulfonate (99% ee).
[0054] Step 2) Synthesis of (S) or (R)-1-benzyl-3-hydroxypiperidine
Embodiment 3
[0055] Embodiment 3: the synthesis of (S)-1-benzyl-3-hydroxypiperidine
[0056] (1) Synthesis of (S)-1-benzyl-3-hydroxypiperidine (1)
[0057] In a 1000ml three-necked bottle, add (S)-1-benzyl-3-hydroxypiperidine camphorsulfonate (99%ee, 84.7 g, 0.2mol), dichloromethane 423ml, 1N aqueous sodium hydroxide solution (210ml) was added dropwise, stirred at room temperature for 1 hour, the water phase was separated, the organic phase was dried over anhydrous sodium sulfate, and concentrated to obtain (S)-1-benzyl -3-hydroxypiperidine 38g. (ee: 99%; LC-MS: m / e=191.3) (theoretical: 38.25 g).
[0058] (2) Synthesis of (S)-1-benzyl-3-hydroxypiperidine (2)
[0059] In a 1000ml three-necked flask, add (S)-1-benzyl-3-hydroxypiperidine camphorsulfonate (99%ee, 84.7 g, 0.2mol), ethyl acetate 423ml, 1N aqueous potassium carbonate solution (150ml) was added dropwise, stirred at room temperature for 1 hour, the water phase was separated, the organic phase was dried over anhydrous sodium sul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com