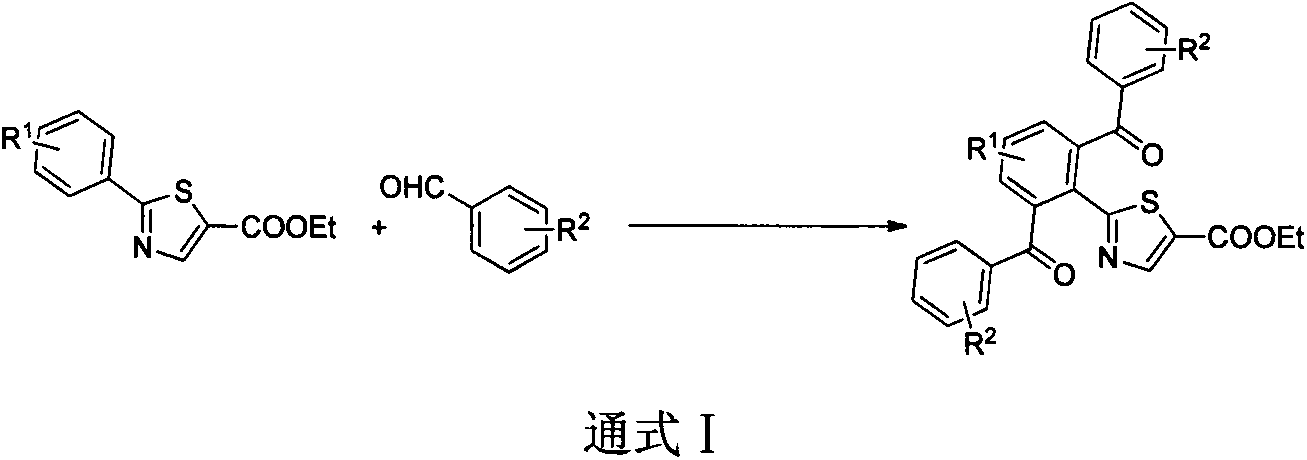
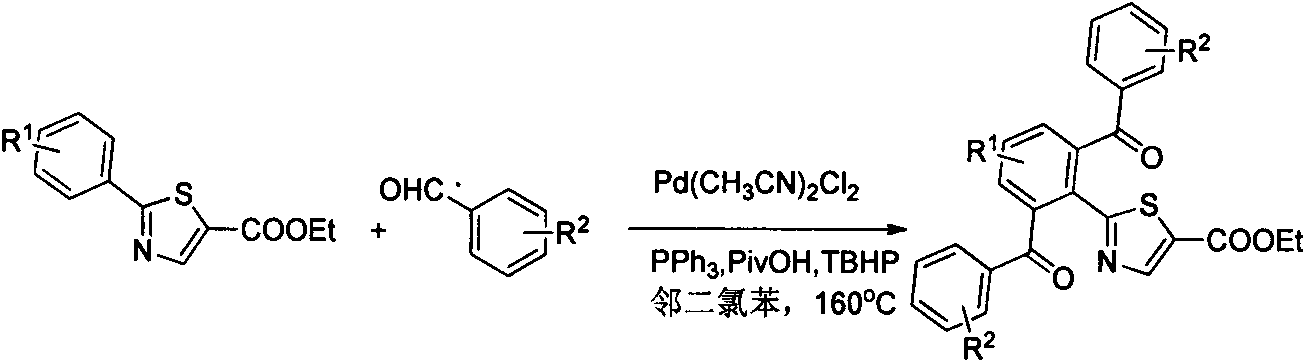
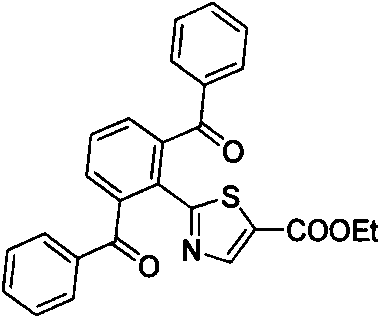
Preparation method of 2-(2,6-dibenzoyl phenyl)thiazole-5-ethyl formate derivative
A technology of dibenzoyl phenyl and ethyl formate, which is applied in the field of preparation of 2-thiazole-5-ethyl formate derivatives, and can solve the problems of lack of new guiding groups and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Ethyl 2-(2,6-dibenzoylphenyl)thiazole-5-carboxylate (1)
[0017]
[0018] Take a reaction tube, add substrate 0.5mmol and substituted benzaldehyde (3mmol, 6eq), diacetonitrile palladium chloride (6.5mg, 0.025mmol, 0.05eq), triphenylphosphine (13mg, 0.05mmol, 0.1eq), Trimethylacetic acid (0.115mL, 1mmol, 2eq), TBHP (65% solution) (0.3mL, 2mmol, 4eq), and o-dichlorobenzene 2mL were reacted in an oil bath at 160°C for 24 hours. The reaction system was cooled to room temperature, diluted with ethyl acetate, and filtered through celite to remove metal residues. The filtrate was subjected to saturated NaOH solution, saturated Na 2 SO 3 solution, washed with saturated brine, anhydrous Na 2 SO 4 Dry and concentrate under reduced pressure to obtain a dark crude product, which is then subjected to silica gel column chromatography to obtain 184 mg of a white solid product with a yield of 83.4%.
[0019] IR (KBr): 3481, 3416, 3134, 3098, 2360, 2331, 1711, 1671, 1587, 1449, ...
Embodiment 2
[0021] Ethyl 2-(2,6-di-p-chlorobenzoylphenyl)thiazole-5-carboxylate (2)
[0022]
[0023] Take a reaction tube, add substrate 0.5mmol and substituted benzaldehyde (3mmol, 6eq), diacetonitrile palladium chloride (6.5mg, 0.025mmol, 0.05eq), triphenylphosphine (13mg, 0.05mmol, 0.1eq), Trimethylacetic acid (0.115mL, 1mmol, 2eq), TBHP (65% solution) (0.3mL, 2mmol, 4eq), and o-dichlorobenzene 2mL were reacted in an oil bath at 160°C for 24 hours. The reaction system was cooled to room temperature, diluted with ethyl acetate, and filtered through celite to remove metal residues. The filtrate was subjected to saturated NaOH solution, saturated Na 2 SO 3 solution, washed with saturated brine, anhydrous Na 2 SO 4 Dry, concentrate under reduced pressure to obtain a dark crude product, and then obtain 175 mg of a white solid product through silica gel column chromatography, with a yield of 68.9%
[0024] IR (KBr): 3418, 3134, 2987, 1724, 1668, 1657, 1585, 1571, 1401, 1309, 1265, 1...
Embodiment 3
[0026] Ethyl 2-(2,6-di-m-chlorobenzoylphenyl)thiazole-5-carboxylate (3)
[0027]
[0028] Take a reaction tube, add substrate 0.5mmol and substituted benzaldehyde (3mmol, 6eq), diacetonitrile palladium chloride (6.5mg, 0.025mmol, 0.05eq), triphenylphosphine (13mg, 0.05mmol, 0.1eq), Trimethylacetic acid (0.115mL, 1mmol, 2eq), TBHP (65% solution) (0.3mL, 2mmol, 4eq), and o-dichlorobenzene 2mL were reacted in an oil bath at 160°C for 24 hours. The reaction system was cooled to room temperature, diluted with ethyl acetate, and filtered through celite to remove metal residues. The filtrate was subjected to saturated NaOH solution, saturated Na 2 SO 3 solution, washed with saturated brine, anhydrous Na 2 SO 4 Dry and concentrate under reduced pressure to obtain a dark crude product, which is then subjected to silica gel column chromatography to obtain 190 mg of a light yellow solid product with a yield of 74.6%.
[0029] IR (KBr): 3475, 3415, 3133, 2360, 2324, 1718, 1681, 16...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com