Orally-disintegrating progesterone tablet and preparation method thereof
A technology for oral disintegrating tablets and progesterone, which is applied in the directions of pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc. Improved medication compliance, fast disintegration, and low side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
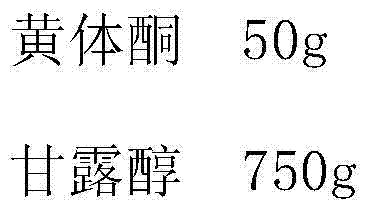
[0022]
[0023]
[0024] Pre-mix the progesterone raw material with mannitol and partially cross-linked polyvinylpyrrolidone in a three-phase granulator; add polyvinylpyrrolidone 50% alcohol aqueous solution to the mixed powder in the previous step to granulate; pour out the wet granules and spread them on a tray, place Dry in an oven at 60oC for 5 hours; sieve through a 26-mesh granule; pour aspartame, orange essence and remaining cross-linked polyvinylpyrrolidone and the granules obtained in the previous step into a three-dimensional mixer and mix for 30 minutes, then add hard Magnesium fatty acid is mixed for 1 to 2 minutes; the mixed material is poured into the hopper of the tablet press, the weight and pressure of the tablet are adjusted, and the tablet is pressed and the hardness of the tablet is kept at 20-40N; double-aluminum cold-formed blister packaging.
Embodiment 2
[0026]
[0027] Pre-mix the progesterone raw material with mannitol and partially cross-linked polyvinylpyrrolidone in a three-phase granulator; add polyvinylpyrrolidone 50% alcohol aqueous solution to the mixed powder in the previous step to granulate; pour out the wet granules and spread them on a tray, place Dry in an oven at 60oC for 4 hours. Sieve through a 26-mesh granule; pour aspartame, orange essence and remaining cross-linked polyvinylpyrrolidone and the granules obtained in the previous step into a three-dimensional mixer and mix for 30 minutes, then add magnesium stearate and mix for 1 to 2 minutes Pour the mixed material into the hopper of the tablet press, adjust the weight and pressure of the tablet, press the tablet and keep the hardness of the tablet at 20-40N; double-aluminum cold-formed blister packaging.
Embodiment 3
[0029]
[0030] Pre-mix the progesterone raw material with mannitol and partially cross-linked polyvinylpyrrolidone in a three-phase granulator; add polyvinylpyrrolidone 50% alcohol aqueous solution to the mixed powder in the previous step to granulate; pour out the wet granules and spread them on a tray, place Dry in an oven at 60oC for 7 hours; sieve through a 26-mesh granule; pour aspartame, orange essence and remaining cross-linked polyvinylpyrrolidone and the granules obtained in the previous step into a three-dimensional mixer and mix for 30 minutes, then add hard Magnesium fatty acid is mixed for 1 to 2 minutes; the mixed material is poured into the hopper of the tablet press, the weight and pressure of the tablet are adjusted, and the tablet is pressed and the hardness of the tablet is kept at 20-40N; double-aluminum cold-formed blister packaging.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com