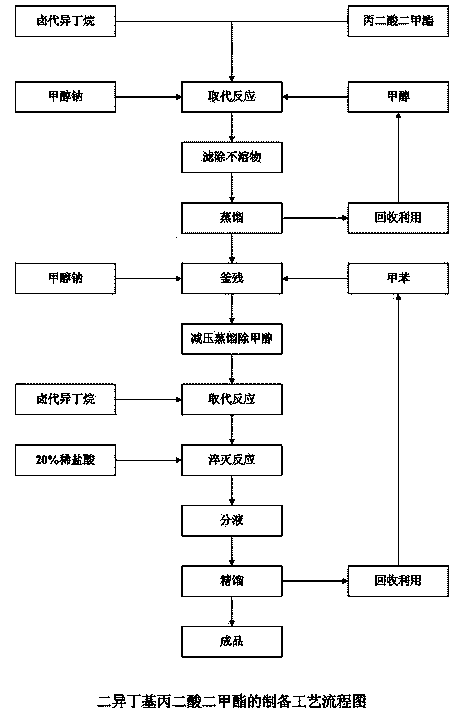
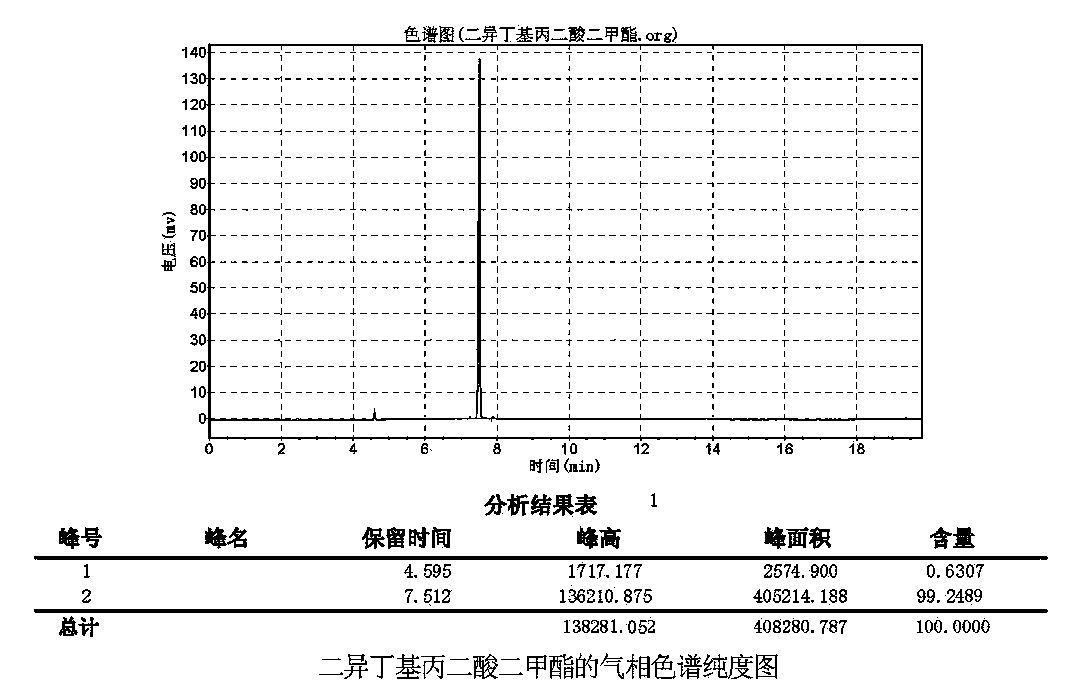
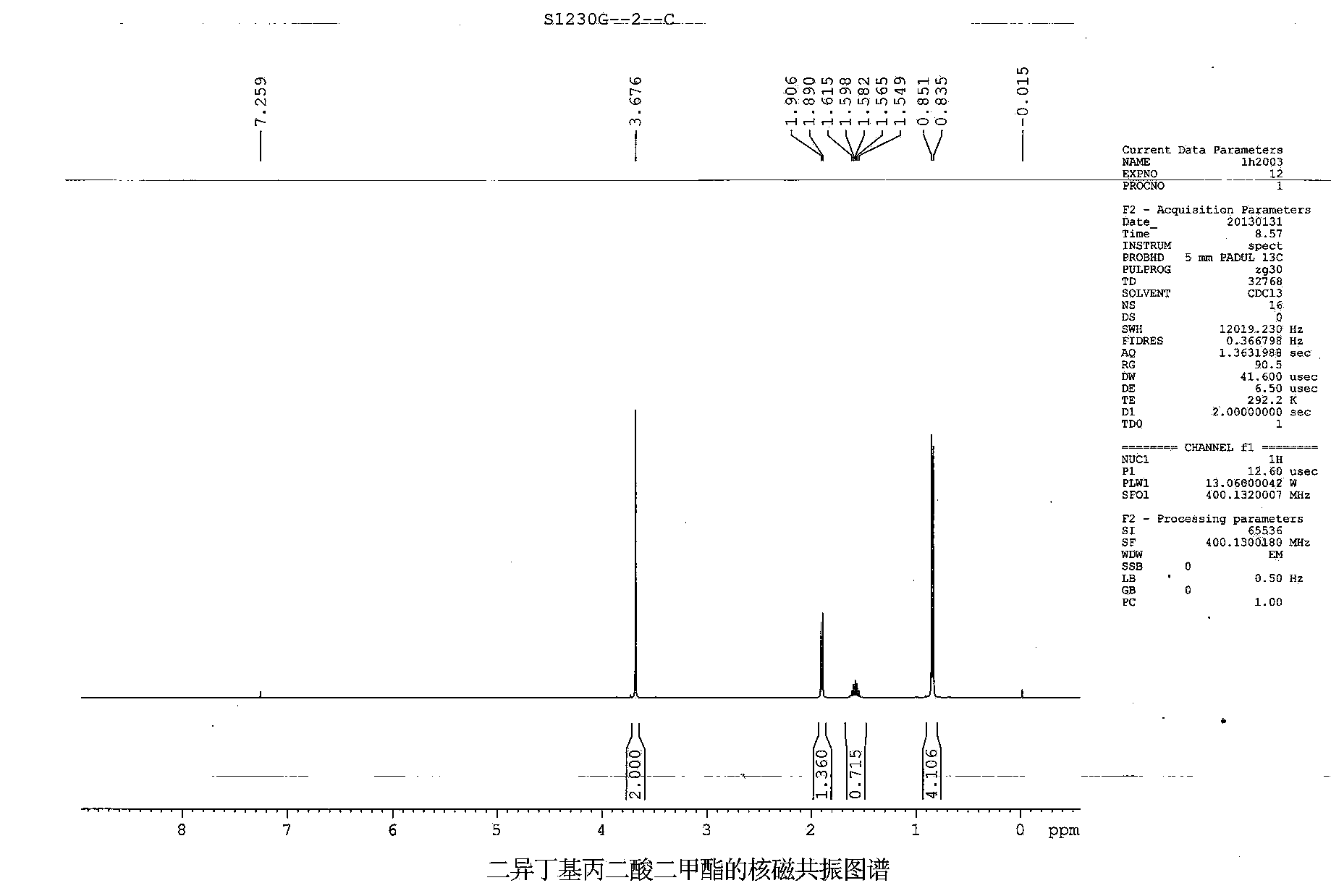
Synthetic method of preparing diisobutyl dimethyl malonate
A technology of diisobutyl dimethyl malonate and dimethyl malonate, applied in the field of synthesis of diisobutyl dimethyl malonate, can solve the problem of diisobutyl dimethyl malonate There are no literature reports on ester preparation methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0010] Example 1, 600 ml of anhydrous methanol was placed in a 1 L four-neck flask equipped with an electric stirrer, a thermometer, a 250 ml constant pressure dropping funnel and a condenser, and 89.1 g (1.65 mol) of sodium methoxide was added and heated to Reflux, quickly add 198 g (1.65 mol) dimethyl malonate dropwise, and react for 3-4 h after the addition, then slowly add 226 g (1.65 mol) bromoisobutane dropwise under slight reflux, dropwise for about 2 h After completion, keep reflux for another 16 h; then recover methanol under reduced pressure. After recovering methanol, add 600 ml of toluene, filter out insoluble matter, add 89.1 (1.65 mol) sodium methoxide, and depressurize to 0.085MPa to distill off the methanol generated by the reaction ; Then slowly drop 226 g (1.65 mol) of bromoisobutane, after about 1 h dropwise, keep reflux for 16 h, cool down to room temperature, slowly drop 20% dilute hydrochloric acid until the pH value reaches 4, separate the liquid , Disti...
Embodiment 2
[0011] Example 2, put 600 ml of anhydrous methanol into a 1 L four-neck flask equipped with electric stirring, a thermometer, a 250 ml constant pressure dropping funnel and a condenser, add 89.1 g (1.65 mol) of sodium methoxide, and heat to Reflux, quickly add 198 g (1.65 mol) dimethyl malonate dropwise, and react for 3-4 h after the addition, then slowly add 226 g (1.65 mol) chloroisobutane dropwise under slight reflux, dropwise for about 2 h After completion, keep reflux for another 16 h; then recover methanol under reduced pressure. After recovering methanol, add 600 ml of toluene, filter out insoluble matter, add 89.1 (1.65 mol) sodium methoxide, and depressurize to 0.085MPa to distill off the methanol generated by the reaction ; Then slowly drop 226 g (1.65 mol) of chloroisobutane, after about 1 h dropwise, keep reflux for 16 h, cool down to room temperature, slowly drop 20% dilute hydrochloric acid until the pH value reaches 4, separate the liquid , Distilled under reduc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com