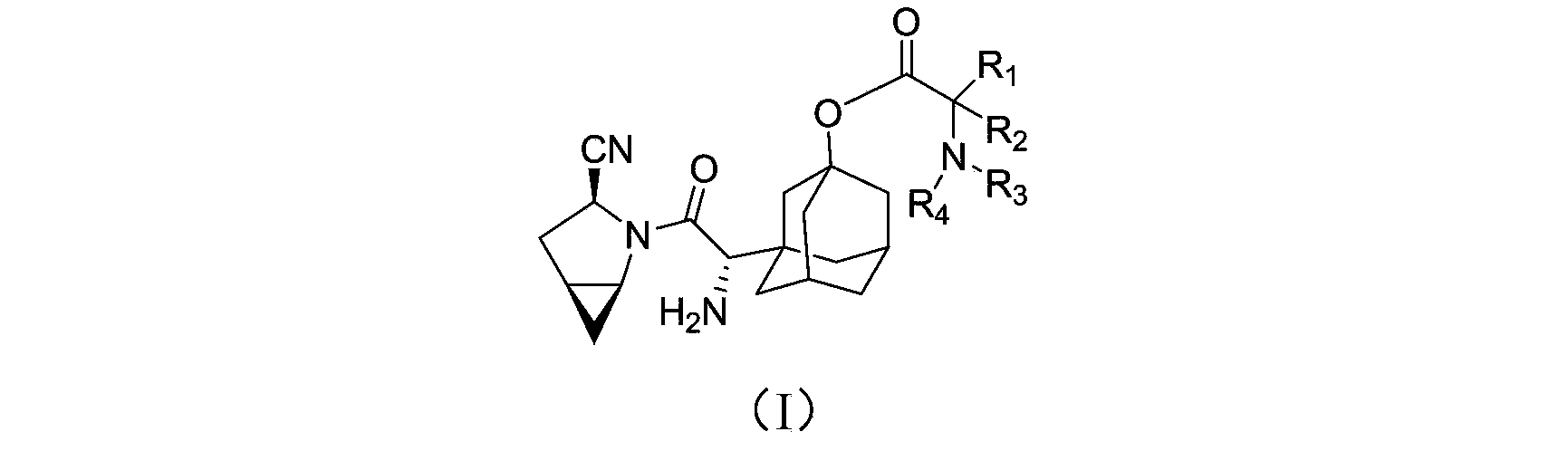
Blood sugar reducing compound, preparation method of blood sugar reducing compound, medicine composition including blood sugar reducing compound and application of medicine composition
A technology of compounds and mixtures, applied in the field of preparation of hypoglycemic drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
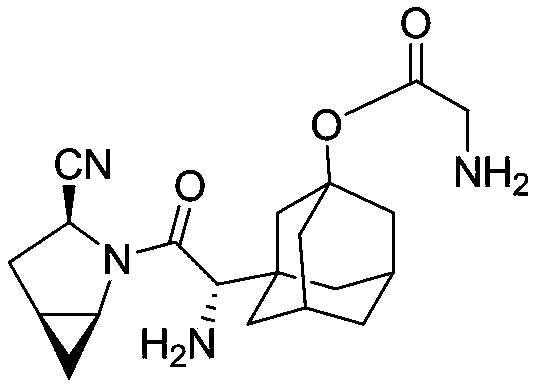
Embodiment 1
[0510] Example 1: Compound 91
[0511]
[0512] ((1S,5S)-1-((1S)-1-amino-2-((3S)-3-nitrile-2-azabicyclo[3,1,0]hexan-2-yl)- 2-oxoethyl)adamantan-3-yl) 2-aminoacetate
[0513] At room temperature, dissolve saxagliptin (4g, 12mmol) in 50ml DMF, add K 2 CO 3 (5g, 36mmol), (Boc) 2 O (5.2g, 24mmol), reacted for 18 hours, added 100ml of dichloromethane, washed four times with water, Na 2 SO 4 Drying and concentration gave 5.4g of the product N-tert-butoxycarbonyl-(1S,3S,5S)-2-[(2S)-2-amino-2-(3-hydroxyl-1-adamantyl)-acetyl Base]-2-azabicyclo[3.1.0]hexane-3-carbonitrile, the compound is C 23 h 33 N 3 o 4 , the calculated value of MS-ESI (m / z) is 415.25; the found value is 438.24 (100, MNa+).
[0514] At room temperature, N-tert-butoxycarbonyl-(1S,3S,5S)-2-[(2S)-2-amino-2-(3-hydroxy-1-adamantyl)-acetyl]-2 -Azabicyclo[3.1.0]hexane-3-carbonitrile (500mg, 1.2mmol) was dissolved in 20ml of dichloromethane, N-Boc-glycine (1053mg, 6.1mmol) was added, DIC (922mg, 7.2mmol) , DMA...
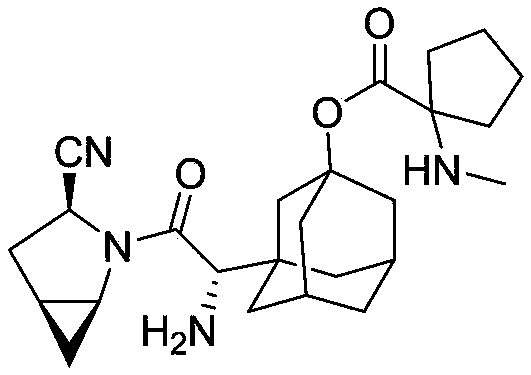
Embodiment 2
[0516] Example 2: Compound 92
[0517]
[0518] (2S)-((1S,5S)-1-((1S)-1-amino-2-((3S)-3-nitrile-2-azabicyclo[3,1,0]hexane-2 -yl)-2-oxoethyl)adamantan-3-yl) 2-aminopropionate
[0519] Prepared as described in Example 1 except that N-tert-butoxycarbonyl-L-alanine was used instead of N-tert-butoxycarbonyl-L-glycine. The resulting compound is C 21 h 30 N 4 o 3 , the calculated value of MS-ESI (m / z) is 386.23; the measured value is 387.13 (100, MH + ).
Embodiment 3
[0520] Example 3: Compound 93
[0521]
[0522] (2R)-((1S,5S)-1-((1S)-1-amino-2-((3S)-3-nitrile-2-azabicyclo[3,1,0]hexane-2 -yl)-2-oxoethyl)adamantan-3-yl) 2-aminopropionate
[0523] Prepared as described in Example 1 except that N-tert-butoxycarbonyl-D-alanine was used instead of N-tert-butoxycarbonyl-L-glycine. The resulting compound is C 21 h 30 N 4 o 3 , the calculated value of MS-ESI (m / z) is 386.23; the measured value is 387.11 (100, MH + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com