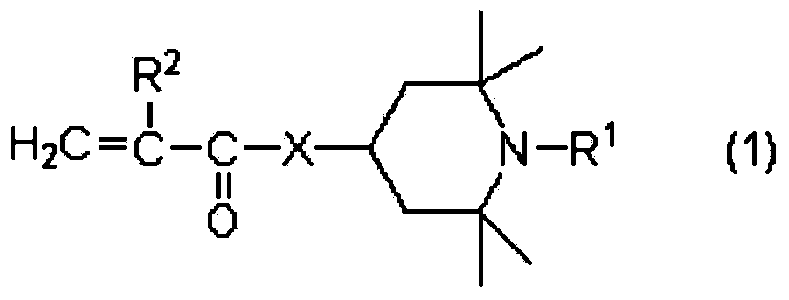
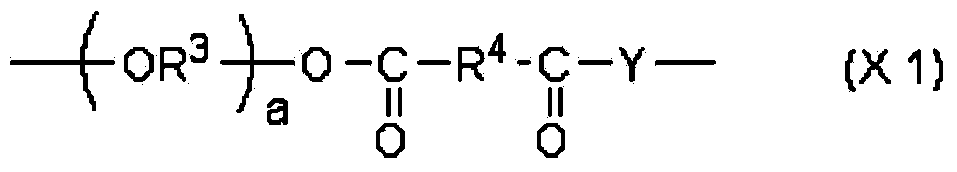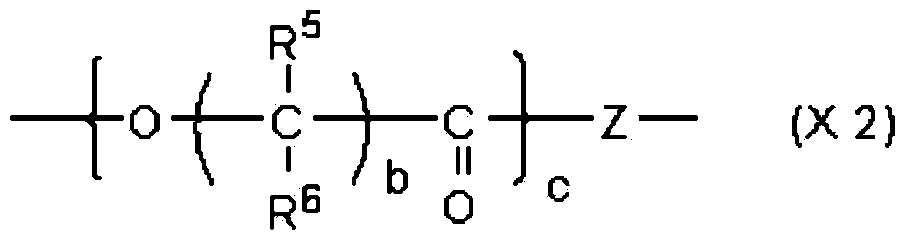(meth)acrylate compound, and photochromic curable composition containing said (meth)acrylate compound
A curable composition, photochromic technology, applied in optics, organic chemistry, optical components, etc., can solve the problems of slow fading speed, lens peeling, blocked lens surface polymerization, etc., to achieve improved effect, high durability, Excellent photochromic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0571] Hereinafter, the present invention will be described in more detail through examples, but the present invention is not limited to these examples.
[0572]
Synthetic example 1
[0574] Synthesis of (1,2,2,6,6-pentamethyl-4-piperidinyl) 2-acryloyloxyethylsuccinate (abbreviation: HM-01):
[0575] Set a stirring blade, a thermometer, and a dropping funnel on a 500mL four-neck flask, and add the following substances.
[0576]
[0577] The mixture was cooled to 0°C, and 12.1 g (0.012 mol) of dicyclohexylcarbodiimide was added little by little. The mixture was stirred at 0 to 5°C for 10 minutes, and further stirred at room temperature for 1 night. After filtering the precipitated white solid, 400 mL of toluene was added to the filtrate, followed by washing with 400 mL of water three times. Furthermore, the organic layer was washed twice with 200 mL of 0.5N hydrochloric acid water, and the washing liquid was collected, neutralized with 5% aqueous sodium carbonate solution, and extracted with toluene. After drying over magnesium sulfate, the solvent was distilled off. The obtained pale yellow liquid was purified with a neutral alumina column {developing...
Synthetic example 2
[0583] Synthesis of acryloyloxypolycaprolactone (c≈2) carboxylic acid (1,2,2,6,6-pentamethyl-4-piperidinyl) ester (abbreviation: HM-02):
[0584] Set a stirring blade, a thermometer, and a dropping funnel on a 500mL four-neck flask, and add the following substances.
[0585]
[0586]
[0587] The mixture was cooled to 0°C, and 12.1 g (0.012 mol) of dicyclohexylcarbodiimide was added little by little. The mixture was stirred at 0 to 5°C for 10 minutes, and further stirred at room temperature for 1 night. After filtering the precipitated white solid, 400 mL of toluene was added to the filtrate, followed by washing with 400 mL of water three times. Furthermore, the organic layer was washed twice with 200 mL of 0.5N hydrochloric acid water, and the washing liquid was collected, neutralized with 5% aqueous sodium carbonate solution, and extracted with toluene. After drying over magnesium sulfate, the solvent was distilled off. The obtained pale yellow liquid was purified w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com