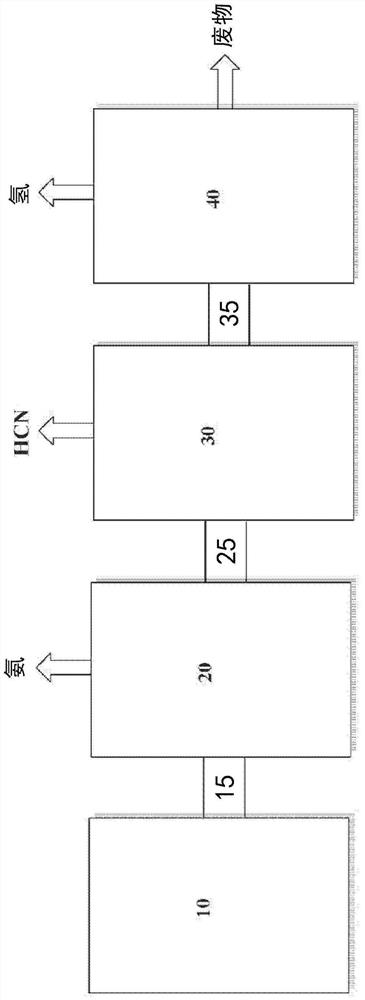
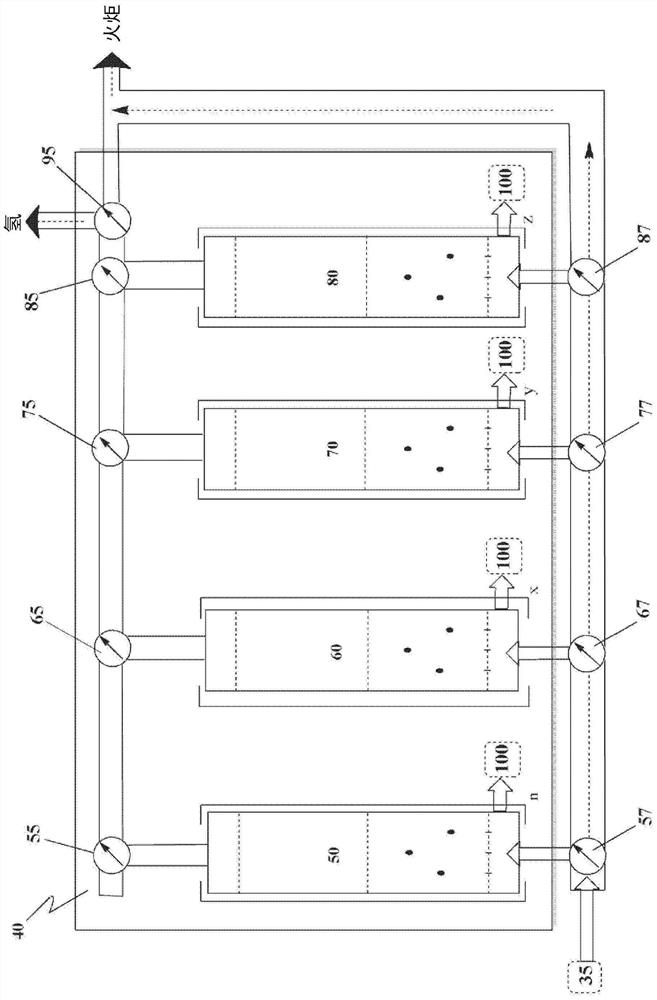
Apparatus and method for hydrogen recovery in the Andrussow process
A hydrogen recovery system, shuttle method technology, applied in the field of hydrogen recovery, can solve the problem of wasting hydrogen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Example 1: Contrasting air and oxygen Andrussow exhaust gas composition
[0100] This example demonstrates that the Andrussow process using a highly enriched source of oxygen generally produces a waste stream with a higher hydrogen content than processes employing air as the oxygen source.
[0101] A 4 inch ID stainless steel reactor with a ceramic insulating liner inside was used for the pilot test. Forty pieces of 90wt%Pt / 10wt%Rh 40 mesh gauze from Johnson Matthey (USA) were loaded as catalyst beds. A perforated alumina sheet was used for the catalyst sheet support. Set the total flow rate at 2532 SCFH (standard cubic feet per hour). Hydrogen cyanide is produced via two independent Andrussow processes. One method is the oxygen Andrussow process using a gaseous reaction mixture comprising 35% by volume methane, 38% by volume ammonia and 27% by volume of substantially pure oxygen. The second method is the air Andrussow method using about 17 vol% methane, 19 vol% amm...
Embodiment 2
[0107] Example 2: Hydrogen content in gaseous waste stream
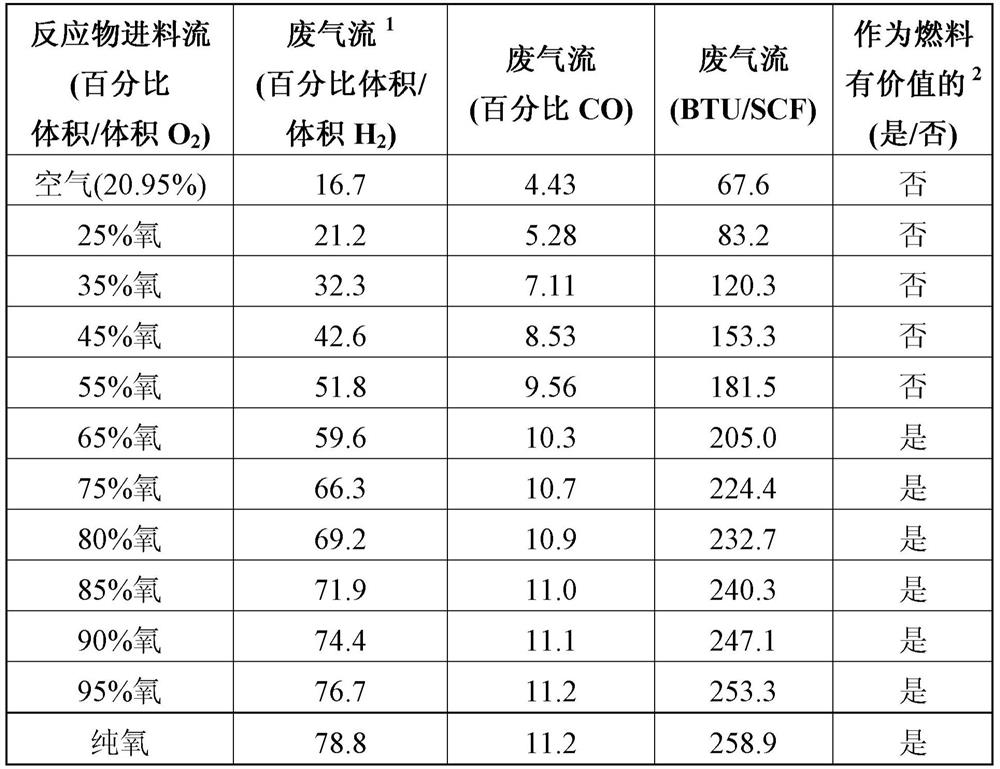
[0108] This example illustrates how the hydrogen content of the gaseous waste stream varies in an Andrussow process using reactants of an oxygen-containing feed stream with different amounts of oxygen.
[0109] Hydrogen cyanide was prepared via a series of separate Andrussow processes performed as described for Example 1 . However, each process was performed using a different reactant oxygen-containing feed stream, wherein the oxygen content of the feed stream varied between about 20.9% v / v to about 100% v / v oxygen, as shown in Table 3 .
[0110] Ammonia is removed from each product stream separately in a process including absorption into the ammonium phosphate stream. Hydrogen cyanide is then removed from the ammonia-lean product stream in a process involving acidification of the water, producing hydrogen cyanide product and gaseous waste streams separately for each process.
[0111] The composition of the gaseou...
Embodiment 3
[0117] Example 3: Hydrogen Recovery by Pressure Swing Adsorption
[0118] This example may illustrate how hydrogen can be recovered from an Andrussow product stream using a pressure swing absorption unit.
[0119] Hydrogen cyanide is produced via the Andrussow process using a gaseous reaction mixture comprising 35 vol% methane, 38 vol% ammonia, and 27 vol% substantially pure oxygen in the presence of a platinum catalyst. A 4 inch ID stainless steel reactor with a ceramic insulating liner inside was used for the pilot test. Forty pieces of 90wt%Pt / 10wt%Rh 40 mesh gauze from Johnson Matthey (USA) were loaded as catalyst beds. A perforated alumina sheet was used for the catalyst sheet support. Set the total flow rate at 2532 SCFH (standard cubic feet per hour). The gaseous product stream from the reactor contained 16.6 vol% hydrogen cyanide, 6.1 vol% unreacted ammonia, 34.5 vol% hydrogen, 6.0 vol% CO and 33.6 vol% H 2 O.
[0120] Ammonia is removed from each product stream s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com