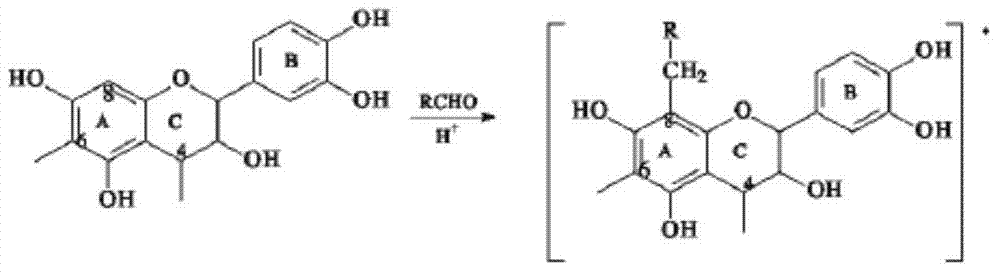
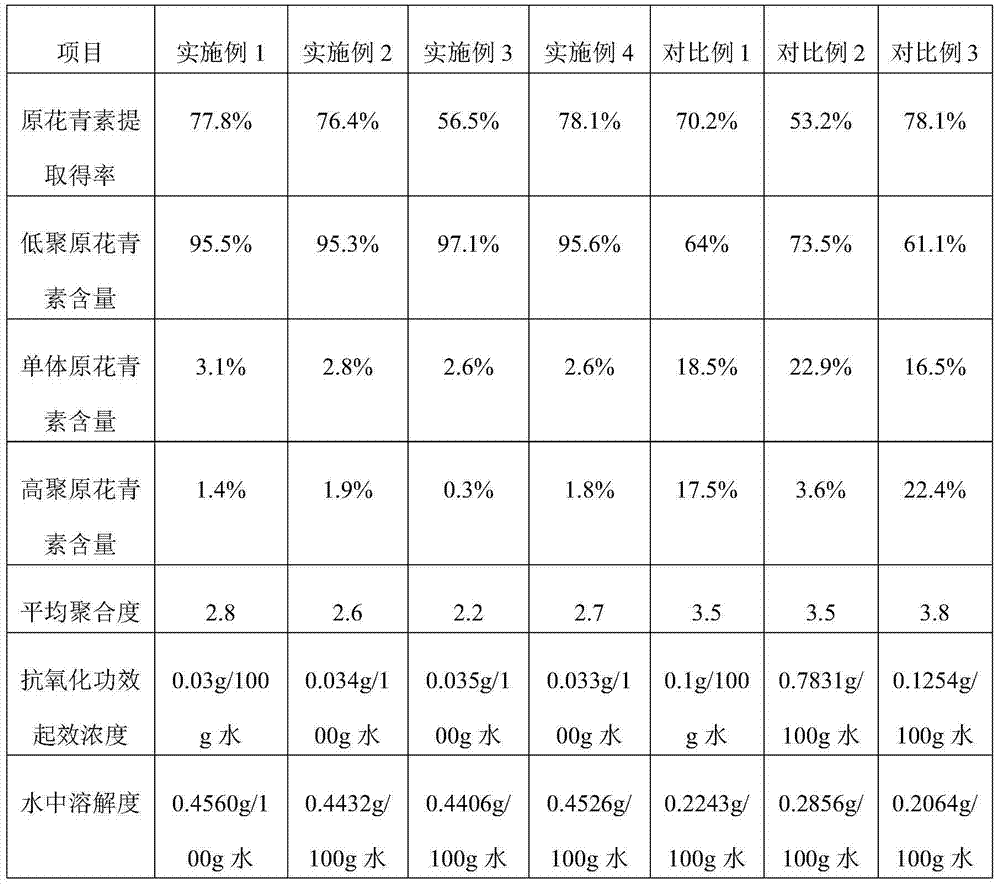
Method for preparing oligomeric proanthocyanidins
A technology of proanthocyanidins and oligomers, applied in the direction of organic chemistry, can solve problems affecting product performance, etc., and achieve the effects of improved solubility, best extraction effect, and reduced content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The oligomer proanthocyanidins described in this embodiment are prepared by the following method:
[0035] (1) Freeze-dry 100 g of fresh grape seeds at -15°C to -5°C and 1 to 10 Pa, then crush them and pass through a 50-mesh sieve to obtain dry powder;
[0036] (2) Take 700ml of ethanol aqueous solution with a volume concentration of 70%, extract the grape seed dry powder at 50°C, and control the pH at 4.0, extract 3 times for 30 minutes each time, combine the extracts and rough filter to obtain the crude proanthocyanidin extract ;
[0037] (3) Concentrate the above extract under reduced pressure at a vacuum degree of 0.09Mpa and a temperature of 40-50°C to a specific gravity of 1.10, and perform adsorption treatment on the obtained concentrated solution on AB-8 macroporous resin for 4 hours; and wash it away with pure water impurities;
[0038] (4) Elute the resin with 5% ethanol-water solution 2BV, collect the eluate to obtain monomeric proanthocyanidins, and discar...
Embodiment 2
[0043] The oligomer proanthocyanidins described in this embodiment are prepared by the following method:
[0044] (1) Freeze-dry 100g of fresh grape seeds at -15°C to -5°C and 1 to 10 Pa, then pulverize them, and pass through a 80-mesh sieve to obtain dry powder;
[0045](2) Take 600ml of ethanol aqueous solution with a volume concentration of 70%, extract the dry grape seed powder at 45°C, and control the pH at about 4.3, extract 3 times for 40 minutes each time, combine the extracts and rough filter to obtain the crude proanthocyanidins. liquid;
[0046] (3) Concentrate the above extract under reduced pressure at a vacuum degree of 0.09Mpa and a temperature of 40-50°C to a specific gravity of 1.15, and apply the AB-8 macroporous resin to the obtained concentrate for 3 hours; and wash away impurities with pure water ;
[0047] (4) Elute the resin with 2BV of ethanol aqueous solution with a volume concentration of 10%, collect the eluate to obtain monomeric proanthocyanidins...
Embodiment 3
[0052] The oligomer proanthocyanidins described in this embodiment are prepared by the following method:
[0053] (1) Freeze-dry 100g of fresh grape seeds at -15°C to -5°C and 1 to 10 Pa, then pulverize them, and pass through a 140-mesh sieve to obtain dry powder;
[0054] (2) Take 650ml of ethanol aqueous solution with a volume concentration of 30%, extract the dry grape seed powder at 40°C, and control the pH at 4.4, extract 3 times for 40 minutes each time, combine the extracts and roughly filter to obtain the crude proanthocyanidin extract ;
[0055] (3) Concentrate under reduced pressure to a specific gravity of 1.15 at a vacuum degree of 0.09Mpa and a temperature of 40-50°C, and perform adsorption treatment on AB-8 macroporous resin on the obtained concentrated solution for 3 hours; and wash away impurities with pure water;
[0056] (4) Elute the resin with 2BV of ethanol aqueous solution with a volume concentration of 8%, collect the eluate to obtain monomeric proantho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com