A kind of zinc reagent and its preparation method, application
A zinc reagent, zinc perchlorate technology, applied in chemical instruments and methods, zinc organic compounds, catalysts for physical/chemical processes, etc. Good industrial application prospect, efficient photocatalytic degradation, good catalytic activity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
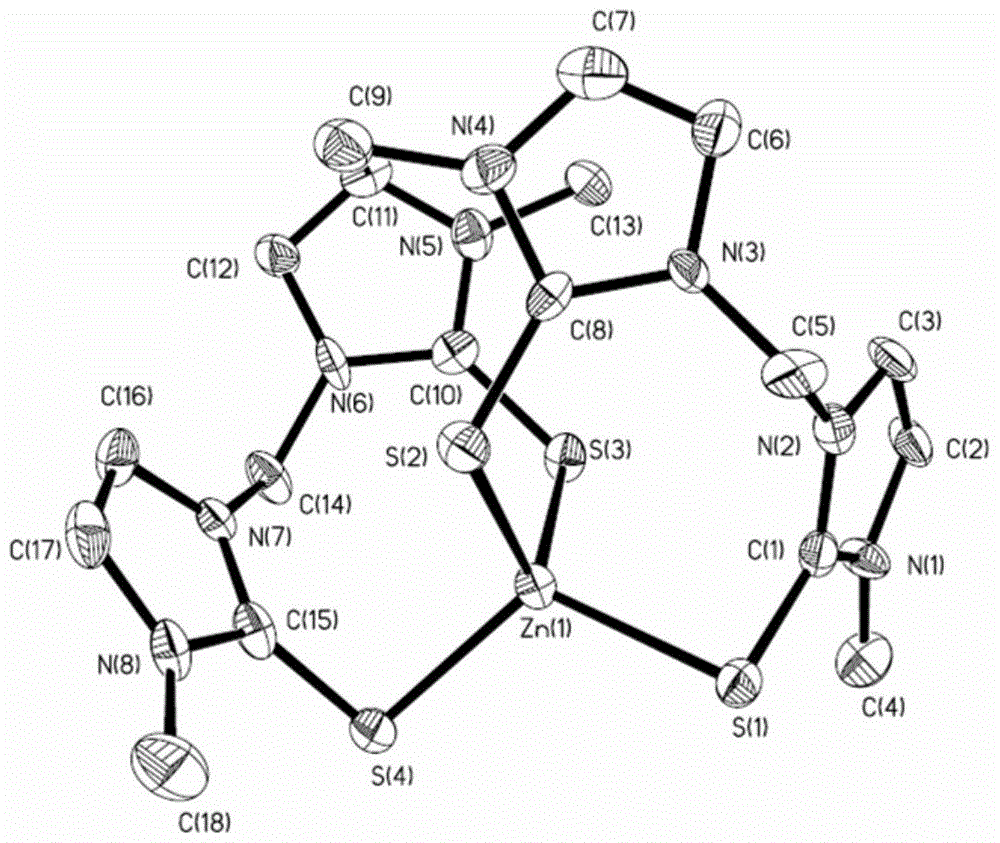
[0027] In a 50ml one-necked flask, add a polytetrafluoroethylene magnetic stirring bar, and add 2mmol zinc perchlorate, 4mmol1,1-bis(1-methylimidazolethione)methane, add 10ml methanol and 10ml dichloro Mix the methane solvent evenly, react for 6 hours at room temperature at 20°C under the protection of nitrogen, a precipitate precipitates out of the solution, filter the filtrate, wash the solid with methanol and ether, and dry to obtain 1.19g (80%) white Solid, 1,1-two (1-methylimidazolium thione) zinc perchlorate coordination compound, that is, zinc reagent. 1 HNMR (300MHz, CD 3 CN): δ(ppm)=3.39(s,2CH 3 ,6H),6.41(s,CH 2 ,2H),7.29(d,imidazole,2H),7.30(d,imidazole,2H),7.57(d,imidazole,2H),7.58(d,imidazole,2H); 13 CNMR(300MHz,CD3CN):δ(ppm)=36.89(CH 3 ), 59.44 (CH 2 ), 118.37 (imidazole), 121.65 (imidazole), 124.36 (imidazole), 152.91 (C=S).
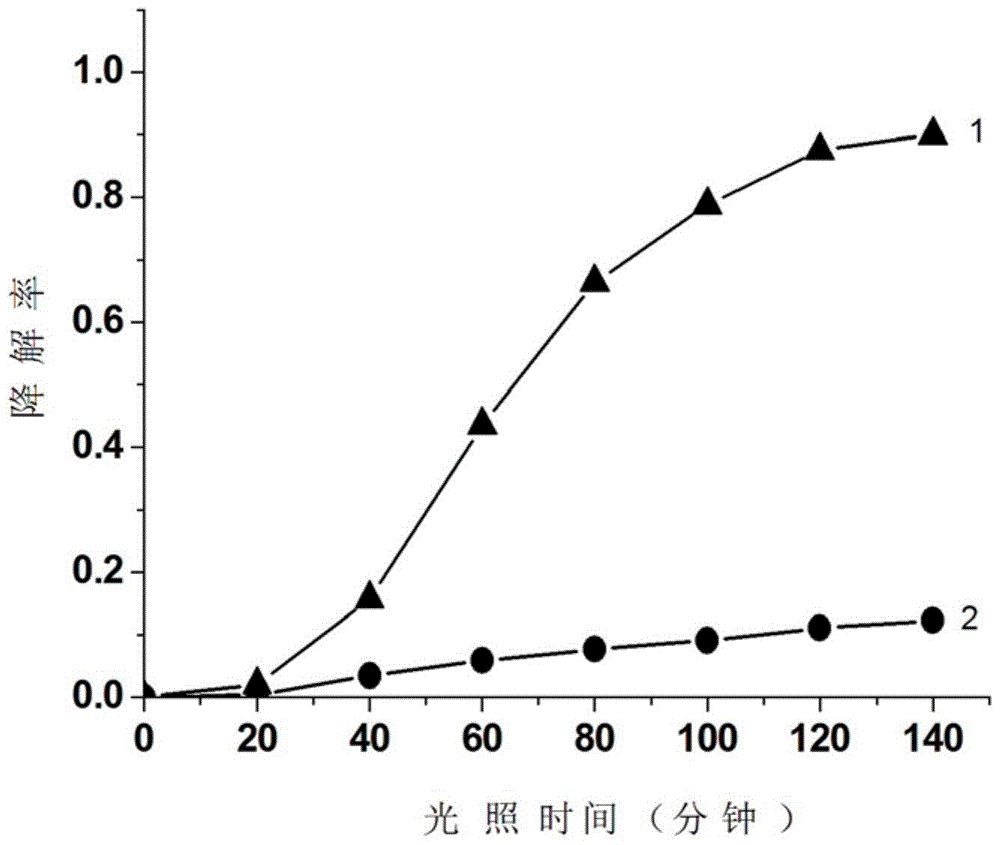
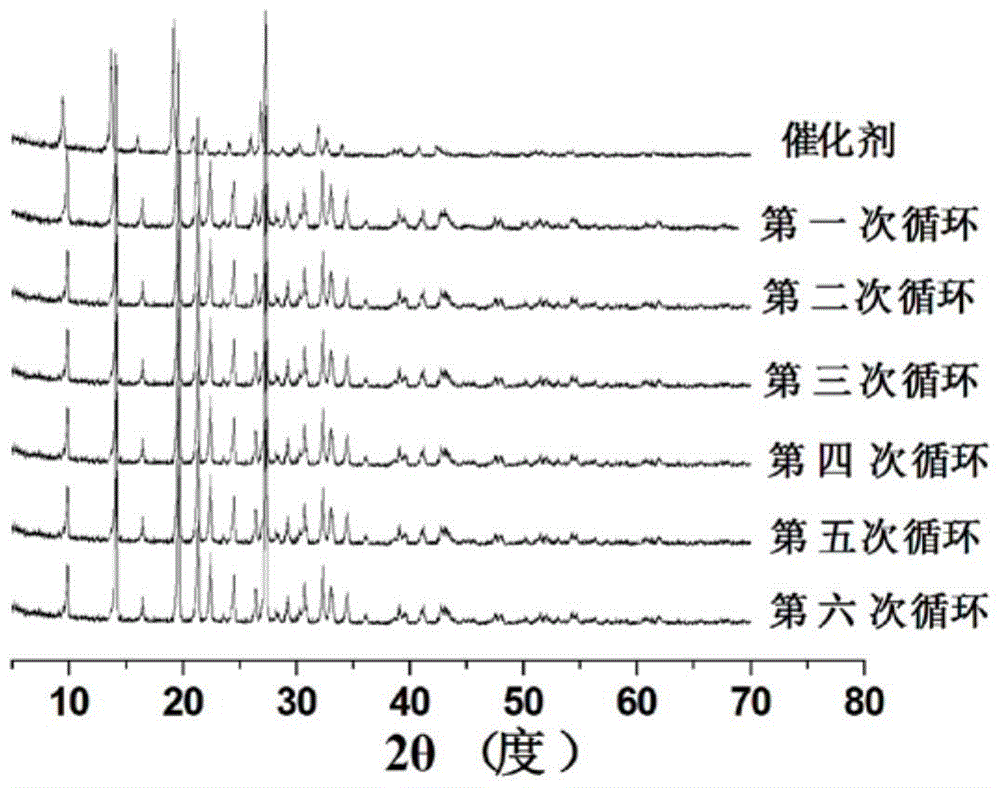
[0028] Take a 100mL beaker, add 150mg of zinc reagent, and then add 400mL of 10mg / L methyl orange solution, ultrasonic for 15 minute...
Embodiment 2
[0035] In a 50ml one-necked flask, add a polytetrafluoroethylene magnetic stirring bar, and weigh 2mmol of zinc perchlorate, 10mmol of 1,1-bis(1-methylimidazolethione)methane, add 10ml of methanol and 10ml of di Mix the methyl chloride solvent evenly, react for 6 hours at room temperature at 30°C under the protection of nitrogen, a precipitate precipitates out of the solution, filter the filtrate, wash the solid with methanol and ether, and dry to obtain 1.10g (79%) white Solid, 1,1-bis(1-methylimidazolethione) zinc perchlorate coordination compound.
Embodiment 3
[0037] In a 50ml one-necked flask, add a polytetrafluoroethylene magnetic stirring bar, and weigh 20mmol of zinc perchlorate, 4mmol of 1,1-bis(1-methylimidazolethione)methane, add 10ml of methanol and 10ml of di Mix the methyl chloride solvent evenly, react for 7 hours at room temperature at 10°C under the protection of nitrogen, a precipitate precipitates out of the solution, filter the filtrate, wash the solid with methanol and ether, and dry to obtain 1.25g (81%) white Solid, 1,1-bis(1-methylimidazolethione) zinc perchlorate coordination compound.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com