Calcium stannate europium luminescence material having hollow structure, and preparation method thereof
A technology of luminescent materials and hollow structures, applied in the direction of luminescent materials, chemical instruments and methods, etc., can solve the problems of inconsistent powder particle size, low luminous intensity of fluorescent powder, high energy consumption of high temperature reaction, etc. The effect of low cost and few process steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
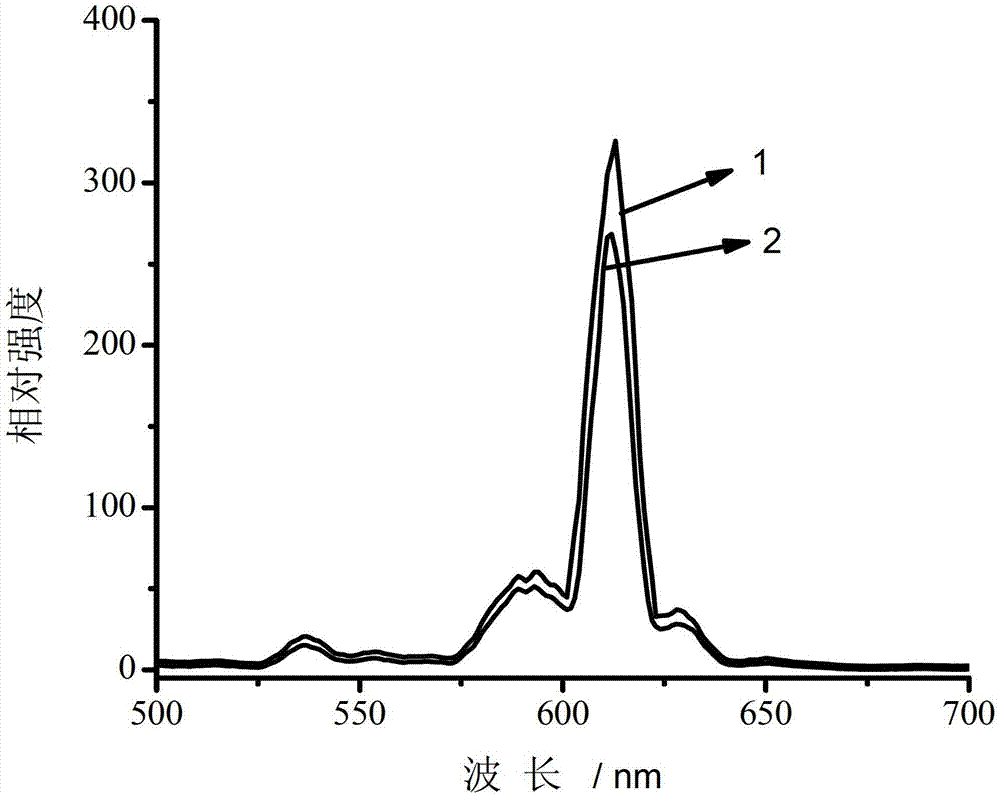
[0026] Precipitation method to prepare Ca with hollow structure 1.95 SnO 4 :Eu 0.05
[0027] Weigh 4g of glucose and dissolve it in absolute ethanol to obtain 40mL of glucitol solution, transfer this solution into a 50mL reaction kettle with a polytetrafluoroethylene liner, cover it and tighten it, and react at 120°C for 36h to prepare carbon small The sphere solution was centrifuged to obtain a solid phase, washed twice with deionized water and absolute ethanol, and dried at 60°C to obtain a carbon sphere template.
[0028] According to Ca 1.95 SnO 4 :Eu 0.05 The stoichiometric ratio measures 1.56mL5mol / L Ca(CH 3 COO) 2 solution, 4mL 0.05mol / L Eu(CH 3 COO) 3 solution and 40mL0.1mol / L SnCl 4 The solution was placed in a beaker, and then 480mg of carbon pellet template was added, heated in a water bath at 50°C, and stirred for 2h. Then slowly add 10mL1mol / L sedimentation agent ammonium oxalate (NH 4 ) 2 C 2 o 4 The solution was kept in a water bath at 50°C for 6h...
Embodiment 2
[0031] Precipitation method to prepare Ca with hollow structure 1.98 SnO 4 :Eu 0.02 :
[0032]Weigh 0.05g of sucrose and dissolve it in absolute ethanol to obtain 40mL of sucrose alcohol solution, transfer the solution into a 50mL reaction kettle with Teflon lining, cover and tighten it, and react at 160°C for 20h to prepare carbon The bead solution was centrifuged to obtain a solid phase, washed three times with deionized water and absolute ethanol, and dried at 80°C to obtain a carbon bead template.
[0033] According to Ca 1.98 SnO 4 :Eu 0.02 The stoichiometric ratio measures 7.92mL1mol / L Ca(NO 3 ) 2 solution, 8mL0.01mol / L Eu(NO 3 ) 3 solution and 8mL0.5mol / L SnCl 4 The solution was placed in a beaker, and then 4.8mg of carbon pellet template was added, heated in a water bath at 80°C, and stirred for 0.5h. Then slowly add 10mL1mol / L sedimentation agent ammonium oxalate (NH 4 ) 2 C 2 o 4 The solution was kept in a water bath at 80°C for 6h. Filter, wash, and ...
Embodiment 3
[0036] Precipitation method to prepare Ca with hollow structure 1.99 SnO 4 :Eu 0.01 :
[0037] Weigh 0.003g of glucose and dissolve it in absolute ethanol to obtain 40mL of glucitol solution, transfer this solution into a 50mL reaction kettle with a polytetrafluoroethylene liner, cover and tighten it, and react at 150°C for 10h to prepare carbon The bead solution was centrifuged to obtain a solid phase, washed twice with deionized water and absolute ethanol, and dried at 70°C to obtain a carbon bead template.
[0038] According to Ca 1.99 SnO 4 :Eu 0.01 The stoichiometric ratio measures 7.96mL1mol / L Ca(NO 3 ) 2 solution, 8mL0.005mol / L Eu(NO 3 ) 3 solution and 4mL1mol / L SnCl 4 The solution was placed in a beaker, and then 200mg of carbon pellet template was added, heated in a water bath at 60°C, and stirred for 1h. Then slowly add 20mL0.5mol / L sedimentation agent ammonium oxalate (NH 4 ) 2 C 2 o 4 The solution was kept in a water bath at 60°C for 3h. Filter, was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com