CRISPR-Cas9 method for specifically knocking out human CCR5 gene and sgRNA for specifically targeting CCR5 gene
A specific and genetic technology, applied in the field of genetic engineering, can solve the problems of ZFN such as time-consuming, expensive, and low efficiency, and achieve good therapeutic effect, improved efficiency, and high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066]Example 1 Design and synthesis of sgRNA for specific targeting of CCR5 gene in CRISPR-Cas9 specific knockout of human CCR5 gene
[0067] 1. Design of sgRNA targeting human CCR5 gene:
[0068] (1) Select the 5'-GGN(19)GG sequence on the CCR5 gene. If there is no 5'-GGN(19)GG sequence, 5'-GN(20)GG or 5'-N(21)GG is also Can.
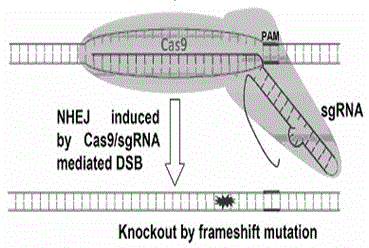
[0069] (2) The targeting site of the sgRNA on the CCR5 gene is located in the exon of the gene, which is more likely to cause fragment deletion or frame-shift mutation, thereby achieving the purpose of complete gene inactivation.
[0070] (3) The targeting site of sgRNA on the CCR5 gene is located on the common exons of different splicing forms.
[0071] (4) Use BLAT in the UCSC database or BLAST in the NCBI database to determine whether the target sequence of the sgRNA is unique and reduce potential off-target sites.
[0072] According to the above method, we designed a total of 100 sgRNAs targeting the human CCR5 gene, the sequences of which are ...
Embodiment 2
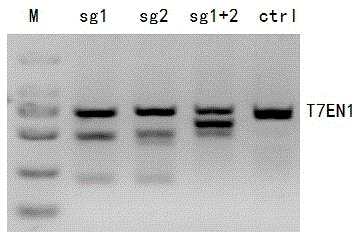
[0089] Example 2 Using CRISPR-Cas9 to specifically knock out the human CCR5 gene (the sgRNA used to target the CCR5 gene is shown in Sequence Table 53)
[0090] 1. The linearized sequence is the pGL3-U6-sgRNA plasmid shown in the sequence table SEQ ID NO.6.
[0091] Enzyme digestion system and conditions are as follows:
[0092] 2 μg pGL3-U6-sgRNA (400ng / μl);
[0093] 1 μl CutSmartBuffer;
[0094] 1 μl BsaI (NEB, R0535L);
[0095] Replenish water to 50 μl, incubate at 37°C for 3-4 hours, shake and centrifuge at intervals to prevent droplets from evaporating onto the tube cap.
[0096] After enzyme digestion, use AxyPrepPCCleanupKit (AP-PCR-250) to purify and recover to 20-40 μl sterilized water.
[0097] 2. The double-stranded sgRNA oligonucleotide obtained after denaturation and annealing that can be connected to the U6 eukaryotic expression vector (its Forwardoligo and Reverseoligo sequences are shown in the sequence table SEQ ID NO.1 and 2, respectively) and the lineari...
Embodiment 3
[0128] Example 3 Using CRISPR-Cas9 to specifically knock out the human CCR5 gene (the sgRNA used to target the CCR5 gene is shown in the sequence table as SEQ ID NO.58)
[0129] 1. The linearized sequence is the pGL3-U6-sgRNA plasmid shown in the sequence table SEQ ID NO.6.
[0130] Enzyme digestion system and conditions are as follows:
[0131] 2 μg pGL3-U6-sgRNA (400ng / μl);
[0132] 1 μl CutSmartBuffer;
[0133] 1 μl BsaI (NEB, R0535L);
[0134] Replenish water to 50 μl, incubate at 37°C for 3-4 hours, shake and centrifuge at intervals to prevent droplets from evaporating onto the tube cap.
[0135] After enzyme digestion, use AxyPrepPCCleanupKit (AP-PCR-250) to purify and recover to 20-40 μl sterilized water.
[0136] 2. The double-stranded sgRNA oligonucleotide obtained after denaturation and annealing that can be connected to the U6 eukaryotic expression vector (its Forwardoligo and Reverseoligo sequences are shown in the sequence table SEQ ID NO.3 and 4, respectively...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com