Myeloma BCMA antigen-targeted transgenic T cell, and preparation method and application thereof
A transgenic and myeloma technology, applied in the direction of targeting specific cell fusion, receptors/cell surface antigens/cell surface determinants, genetically modified cells, etc., can solve the problem of carT cell loss, lack, and treatment efficiency in patients low level problem
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Construction of lentiviral vector
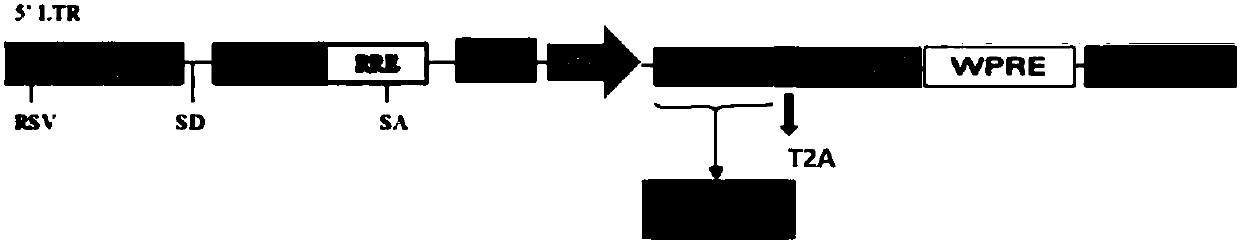
[0038] Schematic diagram of lentiviral vector construction figure 1 shown.
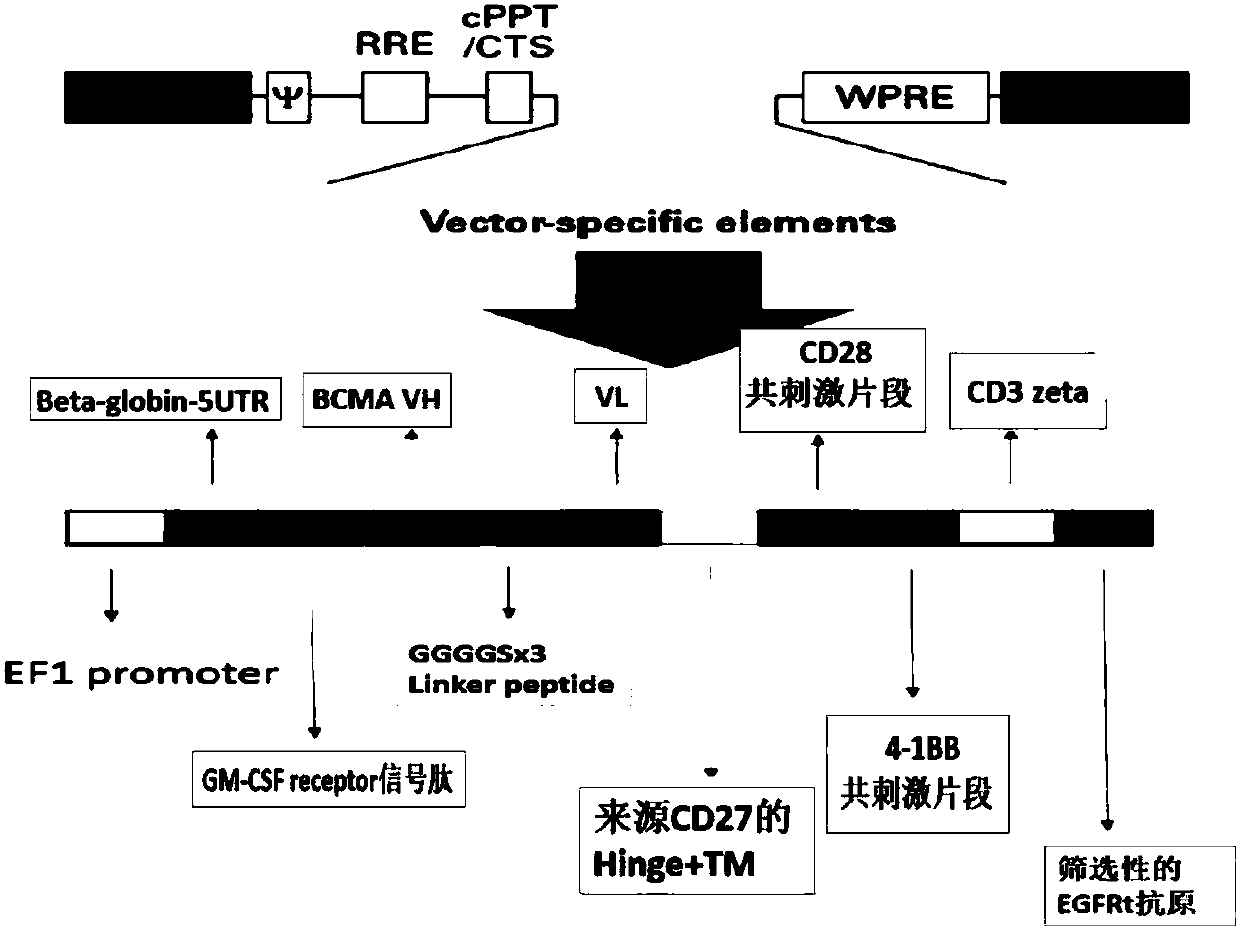
[0039] The design of car molecules and auxiliary screening markers such as figure 2 shown.
[0040] The nucleotide sequence of the constructed car molecule is shown in SEQ ID NO:2.
[0041] Lentiviral coating: a conventional method. Briefly, 293T cells were cultured with RPMI1640+10% FBS. After the cells reached 90% density, the plasmids were mixed and transfected with lipo2000, and the 293T cells were transfected. After the lentiviruses were harvested, they were centrifuged and concentrated. The method of plasmid transfection into 293T cells was in accordance with the reagent guide of lipofactamine 2000 of Invitrogen manufacturer. Plasmids include: lentiviral expression plasmid, 3 kinds of helper plasmids (pMDLg / pRRE, pRSV-Rev, pMD2.G), the molar ratio of mixed plasmids is 2:1:1:0.5. After 24-48 hours of transfection, harvest the supernatant...
Embodiment 2
[0042] Example 2 Culture and lentiviral transfection of T cells
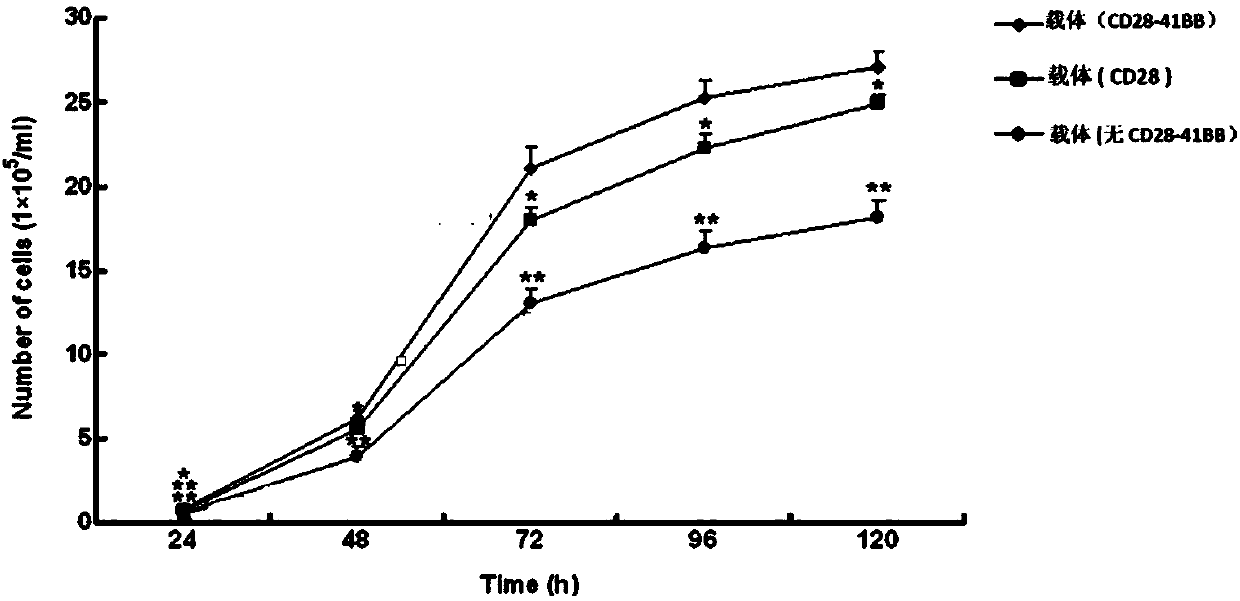
[0043] 50-100 ml of peripheral blood was drawn from healthy people or tumor patients, or mononuclear cells were obtained with a Cobra Spectra blood cell separator, separated by Ficoll, and sorted with CD4+ or CD8+ magnetic beads (the magnetic beads were purchased from Stem Cell Technologies). After T cells were cultured for 1 day (medium formula: Lonza X vivo15, adult serum 10%, IL2300-500IU / ml, penicillin (100units / ml) and streptomycin (100μg / ml), anti-CD3CD28 magnetic beads (with T cell 1:3 mixture) after culturing for 24 hours, they were infected with lentivirus.
[0044] Infection of human CD4+T or CD8+T cells with lentivirus: Refer to the instructions of Takara Retronectin for the prepared and concentrated lentivirus infection, which is briefly described as follows:
[0045] Prepare Retronectin at a concentration of 20-100 μg / ml, and use a density of 4-20 μg / cm for plating 2 After 2 hours at room temperat...
Embodiment 3
[0047] Example 3 Knockout of PD1 gene and CTLA4 gene
[0048] Electrotransfection of CRISPR-cas9 for gene editing of T cells, and experiments for knocking out the PD1 gene and CTLA4 gene respectively, the specific methods are as follows:
[0049] CRISPR-cas9 mRNA and gRNA synthesized in vitro were mixed with HDR, and electroporated into T cells (400V, 0.5ms). Genomic DNA was harvested after the cells were cultured in Lonza X vivo15, adult serum 10%, IL2300-500IU / ml for 3 days. Then do genomic PCR (primers targeting the region near exon2 of PD1, Forward: TTCCTCACCTCTCTCCATCTC; Reverse: CTCTCTTTGATCTGCGCCTT, as shown in SEQ ID NO: 8, SEQ ID NO: 9. Primers targeting the region near exon2 of CTLA4: Forward: TGAGTTCACTGAGTTCCCTTTG; Reverse: GAAATGGCTTTGCTCACCAATTA, as shown in SEQ ID NO: 10, SEQ ID NO: 11), after Agarose gel purification, TA cloning, purification of a single clone and sequencing to obtain Indel mutation information, and calculate the information of the mutated and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com