Ciclopirox olamine vaginal suppository composition and preparation method thereof
A technology of ciclopirox amine and vaginal suppository, which is applied in the field of medicine, can solve the problems of easy stratification, easy occurrence of stratification, and reduced compliance of patients with administration, and achieves the effect of avoiding stratification.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
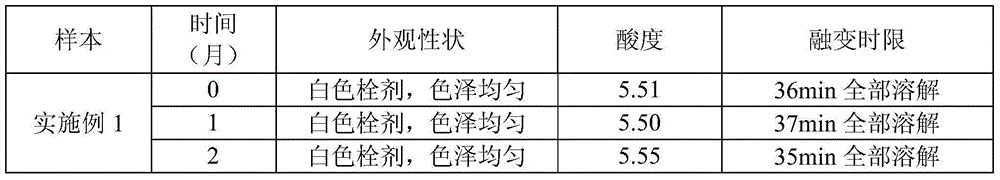
[0046] A ciclopirox olamine vaginal suppository composition is composed of the following components:
[0047] Ciclopirox olamine 100g, polyethylene glycol 40001.6kg, poloxamer 18850g, polysorbate 80250g, citric acid 40g.
[0048] The preparation method of this ciclopirox olamine vaginal suppository composition, concrete steps are as follows:
[0049] Heat 1.6kg polyethylene glycol 4000 and 50g poloxamer 188 to 70°C to melt, add 250g polysorbate 80, stir to mix evenly, add 100g ciclopirox olamine, stir to dissolve, add 40g citric acid and stir Dissolve, mix evenly, cool down to 38°C and keep warm, pack 2.04g each into prefabricated bolt molds, cool and heat seal to get ready.
Embodiment 2
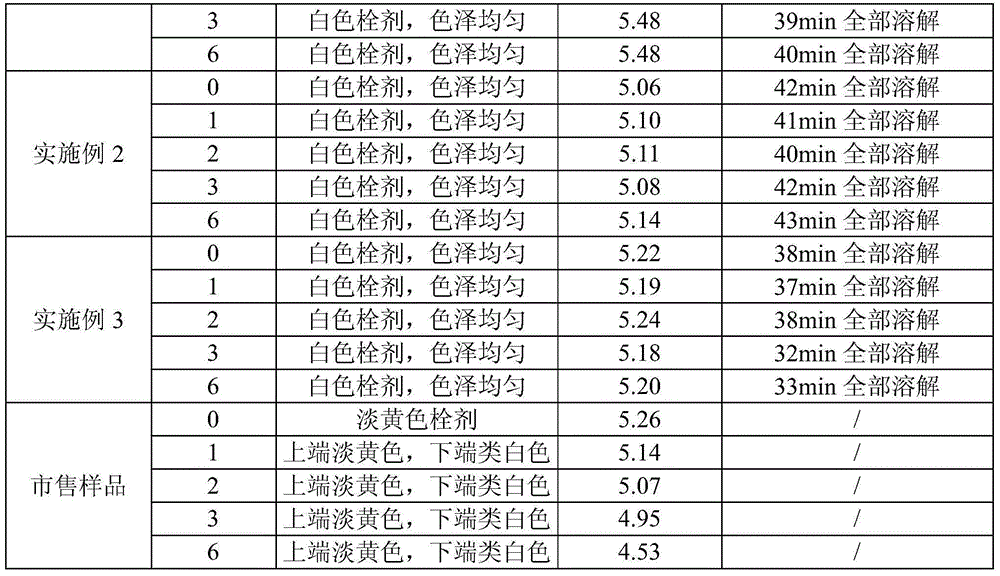
[0051] A ciclopirox olamine vaginal suppository composition is composed of the following components:
[0052] Ciclopirox olamine 100g, polyethylene glycol 60000.9kg, polyethylene glycol 40001.5kg, poloxamer 188110g, polysorbate 80590g, purified water 300g, fumaric acid 50g.
[0053] The preparation method of this ciclopirox olamine vaginal suppository composition, concrete steps are as follows:
[0054] Heat 0.9kg polyethylene glycol 6000, 1.5kg polyethylene glycol 4000, 110g poloxamer 188 to 80°C to melt, add 300g purified water and 590g polysorbate 80, stir to mix evenly, add 100g ciclopirox Stir to dissolve the amine, add 50g of fumaric acid, mix evenly, cool down to 42°C and keep warm, pack 3.55g each into prefabricated plug molds, cool, heat seal, and get ready.
Embodiment 3
[0056] A ciclopirox olamine vaginal suppository composition is composed of the following components:
[0057] Ciclopirox olamine 100g, polyethylene glycol 40002.202kg, poloxamer 18850g, polysorbate 80450g, benzoic acid 48g.
[0058] The preparation method of this ciclopirox olamine vaginal suppository composition, concrete steps are as follows:
[0059] Heat 2.202kg of polyethylene glycol 4000 and 50g of poloxamer 188 to 75°C to melt, add 450g of polysorbate 80 to obtain solution I, dissolve 100g of ciclopirox olamine and 48g of benzoic acid in 500mL of absolute ethanol, and place in a water bath Heat and evaporate ethanol to obtain transparent viscous solution II, mix solution I and solution II evenly, cool down to 40°C and keep warm, pack 2.85g each into prefabricated plug molds, cool, and heat seal to obtain.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com