Tryptophan hydroxylase-1 inhibitor ursolic acid derivatives as well as preparation method and application thereof
A technology of tryptophan hydroxylase and ursolic acid, which is applied in the direction of steroids, organic chemistry, drug combination, etc., to increase the bone density of the spine and prevent osteoporosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The preparation of compound 2, reaction formula is:
[0042]
[0043]Weigh 13.25g (28.8mmol) ursolic acid and dissolve it in 150.0mL N,N-dimethylacetamide or N,N-dimethylformamide at 90°C, slowly add 10.0mL hydrogen peroxide / sodium tungstate / Disodium hydrogen phosphate / sodium dihydrogen phosphate (12.5 / 0.5 / 0.2 / 0.2, molar equivalent) mixed solution, control the dropping time at about 1 hour. After reacting for one hour, it was cooled, poured into cold water, extracted with ethyl acetate, washed, dried over anhydrous sodium sulfate, and evaporated to obtain 3.92 g of 3-carbonyl ursolic acid 2, yield: 86%. Product characterization, the data are: 1 H NMR (300MHz, CDCl 3 )δ: 5.30(s, 1H), 2.76(d, J=15.0Hz, 1H), 2.28–2.14(m, 3H), 2.09–1.97(m, 2H), 1.84–1.69(m, 3H), 1.64 –1.47(m, 6H), 1.33(d, J=2.8Hz, 3H), 1.31(s, 2H), 1.28(d, J=2.1Hz, 3H), 1.23(s, 3H), 1.18(s, 3H), 0.99(s, 3H), 0.97(s, 2H), 0.95(s, 4H), 0.83(s, 3H). 13 CNMR (75MHz, CDCl 3 )δ: 178.1, 142.4, 138.9, 134...
Embodiment 2
[0443] Embodiment 2 ursolic acid derivative concrete experimental example:
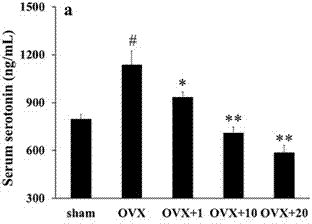
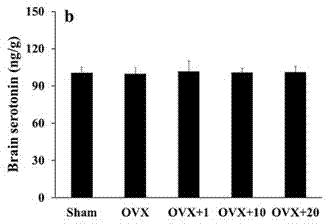
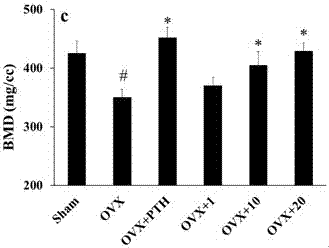
[0444] 1) In vitro inhibition of 5-HT synthesis experiment
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com