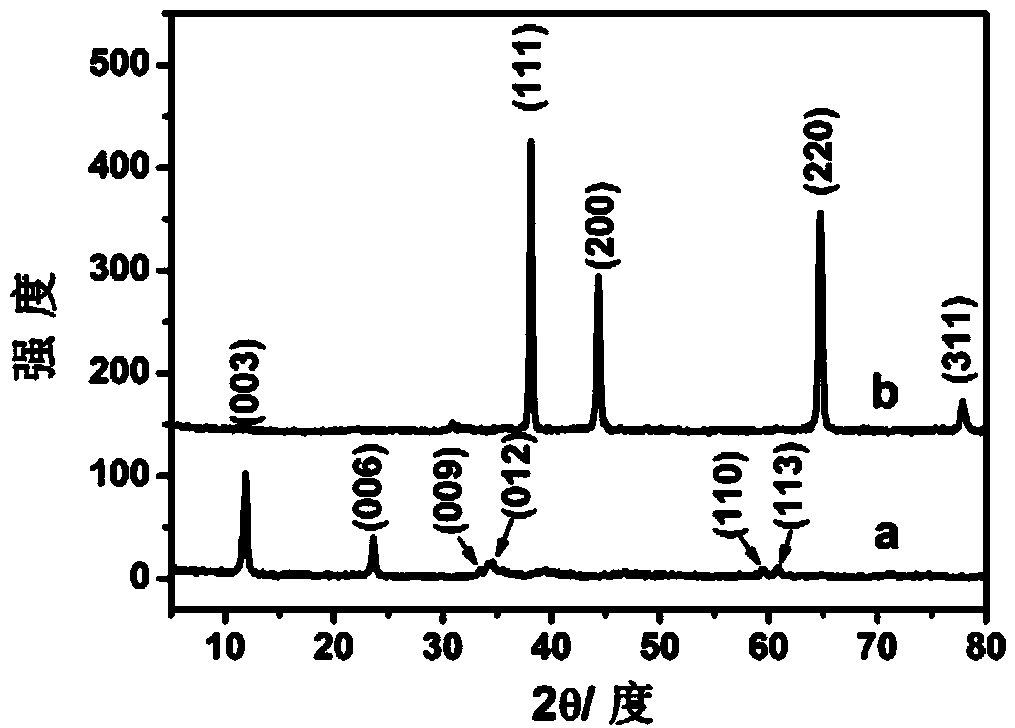
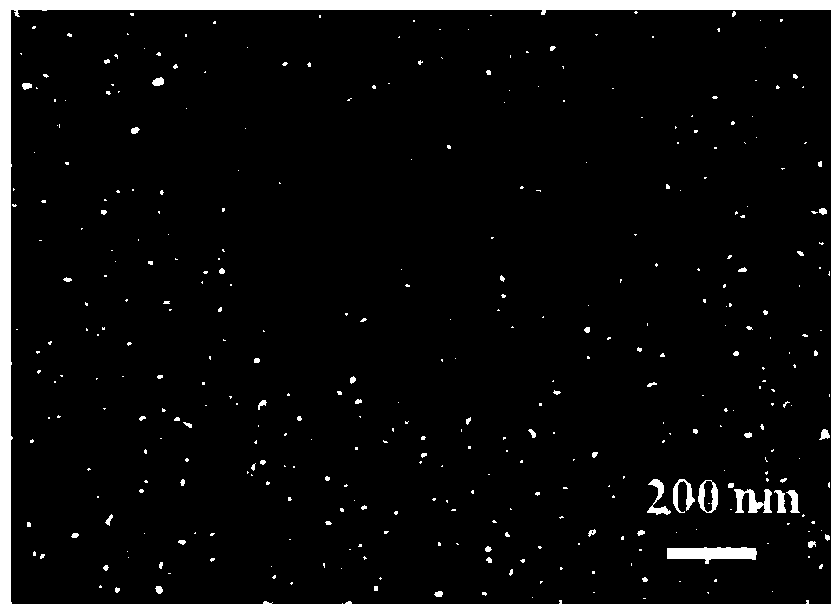
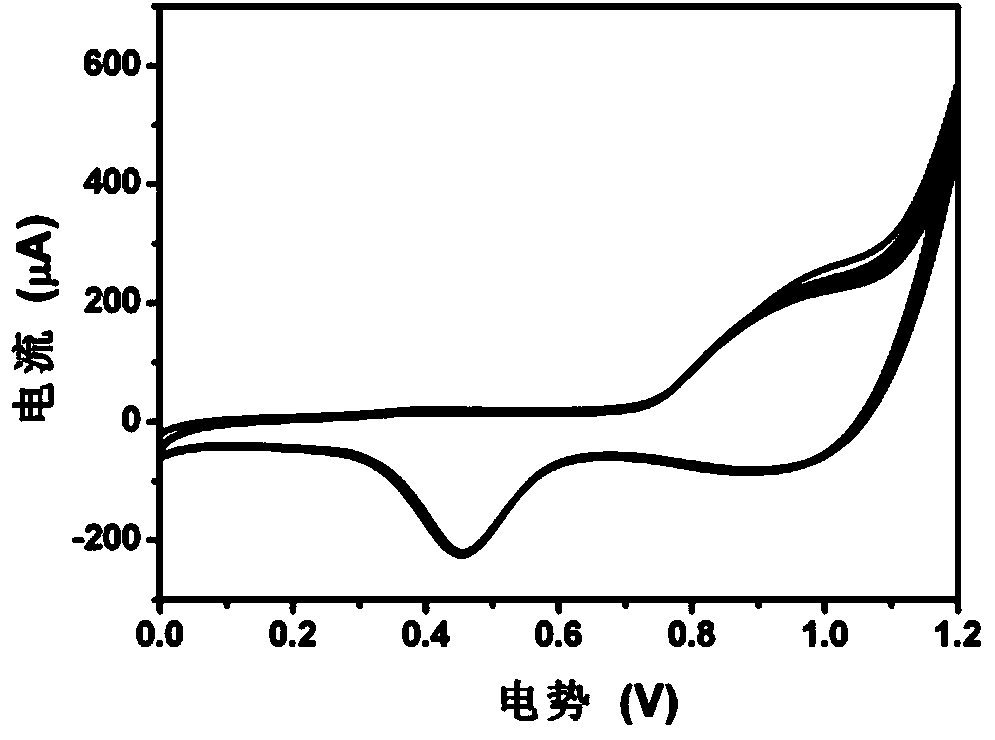
Cobalt-manganese hydrotalcite-supported nanometer gold catalyst and preparation method thereof
A technology of hydrotalcite and catalyst, which is applied in the field of electrocatalyst to achieve the effects of high stability, uniform distribution and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] 1. Carrier Co 3 Preparation of Mn-LDHs
[0021] 3:1 cobalt-manganese hydrotalcite was prepared by nucleation crystallization isolation method, and 4.3658g Co(NO 3 ) 2 ·6H 2 O and 1.7895gMn(NO 3 ) 2 (49%~51%) dissolved in 100mL deionized water to make a mixed salt solution, weigh 1.28g NaOH and 1.06g NaOH 2 CO 3 Dissolve in 100mL deionized water to make a mixed alkali solution. Add the two mixed solutions into the fully back-mixed liquid membrane reactor at the same time, react for 2 minutes, transfer the obtained mixed slurry to a three-necked flask with constant temperature and vigorously stir, crystallize at 30°C for 5 hours, take out the slurry, and wash it with deionized water until the pH is 7 , dried at 50°C for 12 hours to obtain Co 3 Mn-LDHs.
[0022] 2.Co 3 Preparation of Mn-LDHs / AuNPs catalyst
[0023] Take 1mg Co 3 Mn-LDHs powder was dispersed in 1 mL of 2:1 (v / v) isopropanol:water mixed solution and ultrasonically dispersed for 2 hours to obtain ...
Embodiment 2
[0027] 1. Carrier Co 2 Preparation of Mn-LDHs
[0028]2:1 cobalt-manganese hydrotalcite was prepared by nucleation crystallization isolation method, and 38.809g Co(NO 3 ) 2 ·6H 2 O and 23.86g Mn(NO 3 ) 2 (49%~51%) dissolved in 200mL deionized water to make a mixed salt solution, weigh 8g NaOH and 21.2g NaOH 2 CO 3 Dissolve in 200mL deionized water to make a mixed alkali solution. Add the two mixed solutions into the total back-mixed liquid membrane reactor at the same time, react for 1min, transfer the obtained mixed slurry to a three-necked flask with constant temperature and vigorously stir, crystallize at 20°C for 10 hours, take out the slurry, and wash it with deionized water until the pH is 7.1 , dried at 100°C for 24 hours to obtain Co 2 Mn-LDHs.
[0029] 2.Co 2 Preparation of Mn-LDHs / AuNPs catalyst
[0030] Take 2mg Co 2 Mn-LDHs powder was dispersed in 1 mL of 3:1 (v / v) isopropanol:water mixed solution and ultrasonically dispersed for 3 hours to obtain 2 mg / ...
Embodiment 3
[0034] 1. Carrier Co 4 Preparation of Mn-LDHs
[0035] 4:1 cobalt-manganese hydrotalcite was prepared by nucleation crystallization isolation method, and 23.2824g Co(NO 3 ) 2 ·6H 2 O and 3.579g Mn(NO 3 ) 2 (49%~51%) dissolved in 200mL deionized water to make a mixed salt solution, weigh 12g NaOH and 2.12g NaOH 2 CO 3 Dissolve in 200mL deionized water to make a mixed alkali solution. Add the two mixed solutions into the total back-mixed liquid membrane reactor at the same time, react for 2 minutes, transfer the obtained mixed slurry to a three-necked flask with constant temperature and vigorously stir, crystallize at 50°C for 6 hours, take out the slurry, and wash it with deionized water to the pH of the supernatant is 7.5, dried at 60°C for 18 hours to obtain Co 4 Mn-LDHs.
[0036] 2.Co 4 Preparation of Mn-LDHs / AuNPs catalyst
[0037] Take 6mg Co 4 Mn-LDHs powder was dispersed in 2 mL of 4:1 (v / v) isopropanol:water mixed solution and ultrasonically dispersed for 4 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com