Ophthalmic compositions comprising prostaglandin f2α derivatives and hyaluronic acid
一种透明质酸、前列腺素的技术,应用在治疗高眼压症和青光眼领域,能够解决没有销售前列腺素类似物组合物等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0181] Embodiment 1: Eye Drops for Eyes
[0182] Various ophthalmic eye drops are the preferred compositions described herein. However, such examples should in no way be construed in a limiting sense, but rather, as far as possible, are recognized as means by which eventual modifications can be made without departing from the principles of the invention.
[0183] Example of a protocol for preparing a solution comprising a prostaglandin analogue:
[0184] Add disodium hydrogen phosphate anhydrate and sodium dihydrogen phosphate dihydrate to the solution of sodium chloride. After stirring for 10 minutes, the pH of the solution referred to as "Solution 1" was measured and adjusted to 6.3-7.1 using 10% disodium hydrogen phosphate solution or 10% sodium dihydrogen phosphate solution. Then, hyaluronic acid was added to the 10% solution 1. The resulting mixture was stirred for 30 minutes or until completely dissolved, yielding "Solution 2", and cooled at least at 4-8°C. Thereaf...
Embodiment 2
[0203] Example 2: Chemical Stability Study
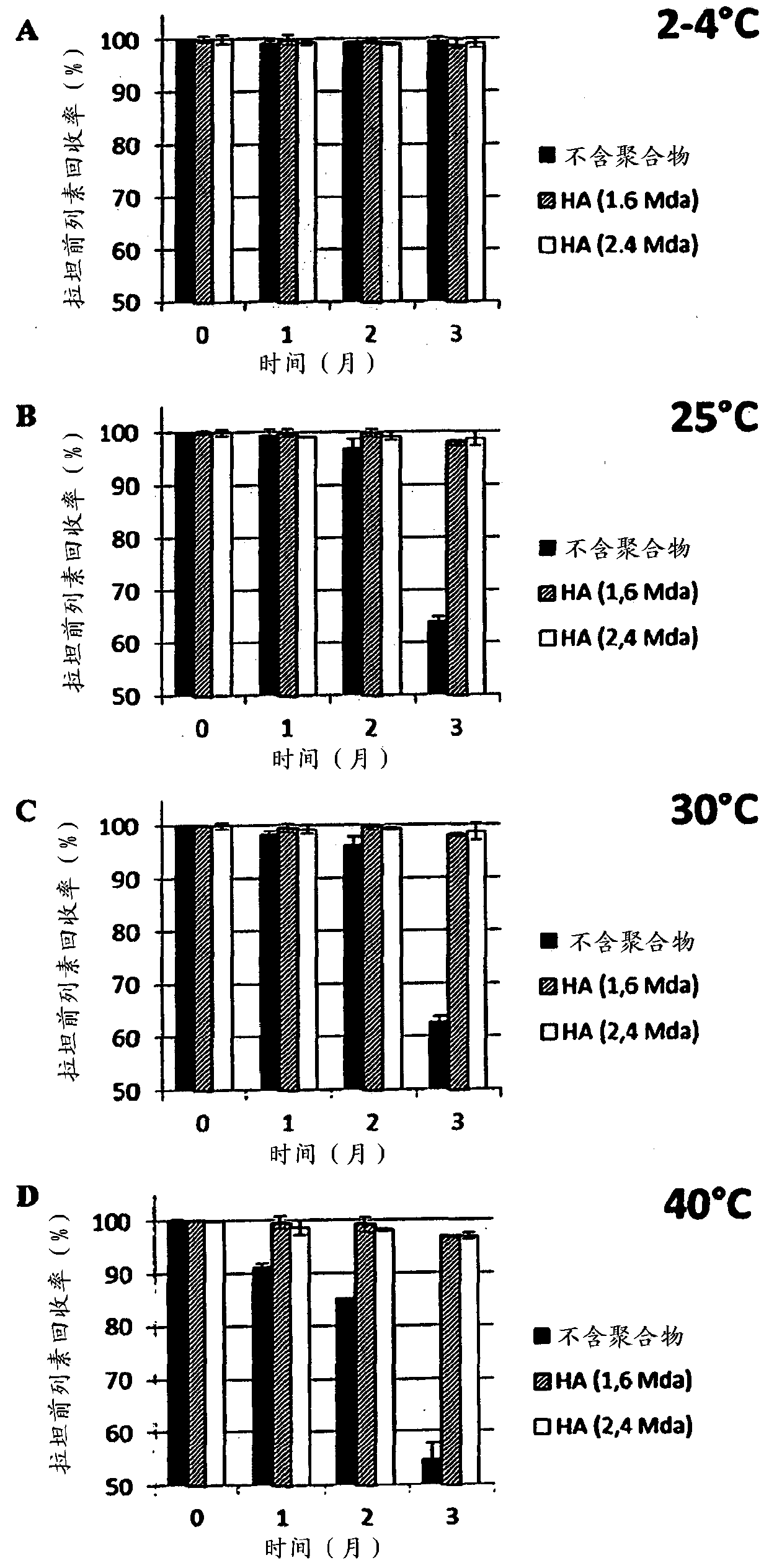
[0204] In order to measure the intrinsic stability of the prostaglandin analogues in the compositions of the present invention, the applicant carried out a stability test in which the compositions of the present invention were subjected to a specific temperature (up to 40° C.) for a specific period of time. Stabilize the samples at different temperatures: refrigerated at 2-4°C (long-term stability test), 25°C (accelerated stability test), 30°C (stress stability test 1) and 40°C (stress stability test 2) sex studies.
[0205] Evaluation of recovery of latanoprost, travoprost and bimatoprost was performed using high pressure liquid chromatography (HPLC) with LC9 pump. In short, samples are kept in glass containers. The content of latanoprost, travoprost and bimatoprost was determined using HPLC equipped with a UV detector and using a mixture of two mobile phases and using Shimadzu Type-VP software.
[0206] The results are presen...
Embodiment 3
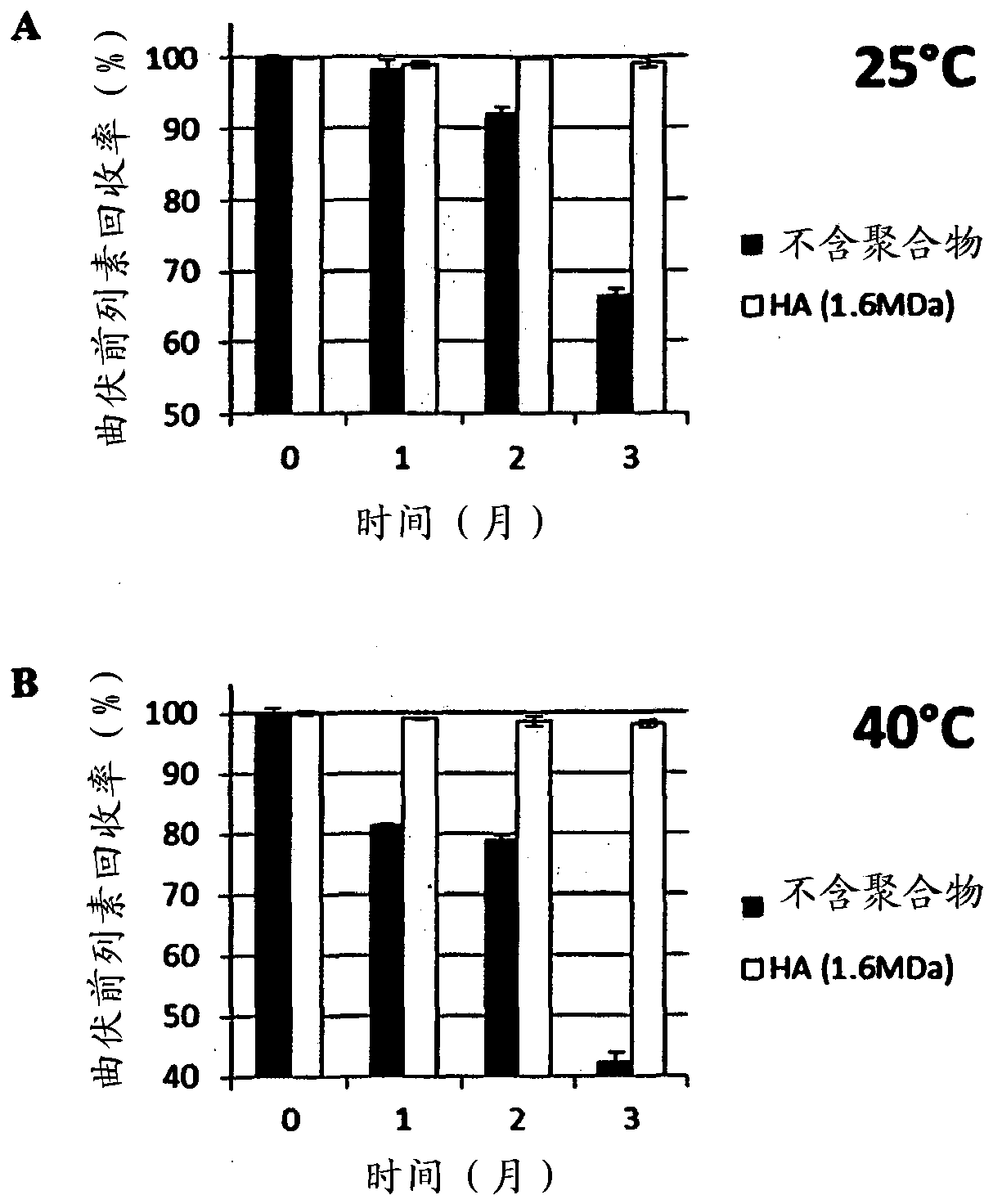
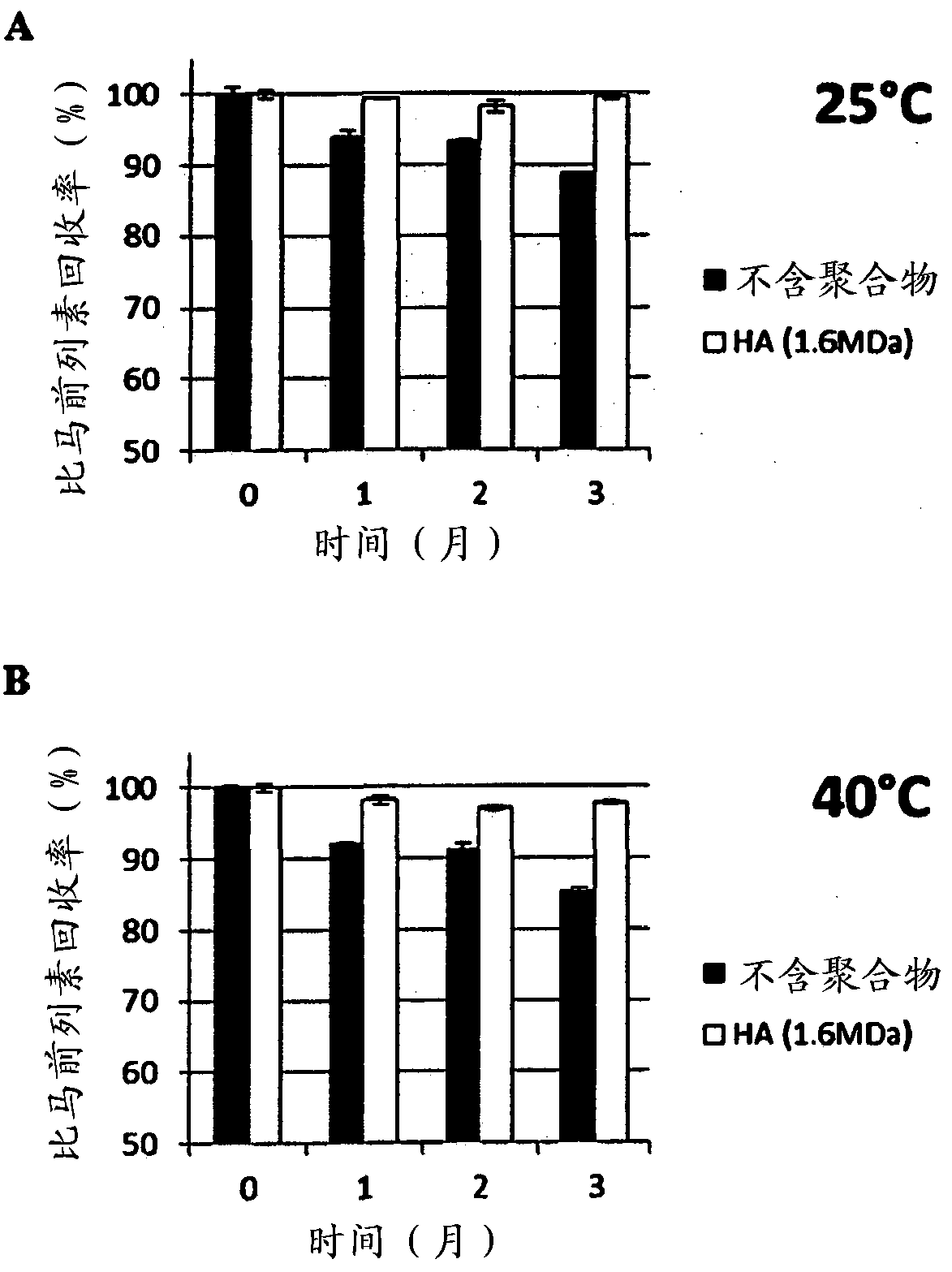
[0213] Example 3: Chemical Stability of Prostaglandins in the Presence of a Range of Molecular Weight Hyaluronic Acid
[0214] Prostaglandin stability was studied using a range of hyaluronic acid molecular weights (60 to 3 million Daltons (MDa)). The test composition contained prostaglandin (0.005% latanoprost or 0.004% travoprost or 0.03% bimatoprost) and 0.15% (w / v) hyaluronic acid in phosphate buffered saline. The recovery of latanoprost, travoprost and bimatoprost was evaluated by high pressure liquid chromatography (HPLC). Briefly, samples were stored in glass containers at 40 °C for 3 months. The content of latanoprost, travoprost and bimatoprost was determined using HPLC equipped with UV detector and using a mixture of two mobile phases and using Shimadzu Type-VP software.
[0215] Results are expressed as the percentage of prostaglandin recovery at 3 months (t=3 months) compared to the initial concentration of prostaglandin (ie, the percentage of prostaglandin reco...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com