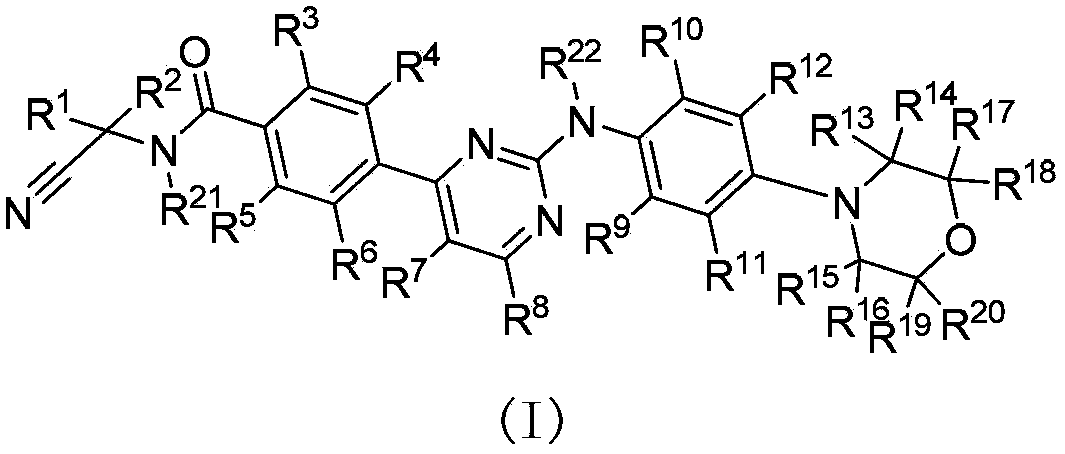
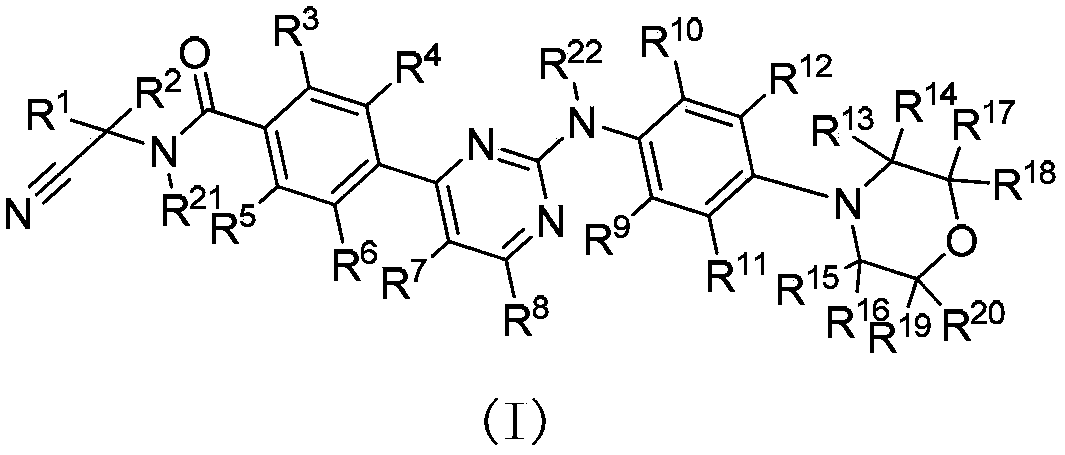
Deuterated phenylamino pyrimidine compound and drug composition containing the same
一种化合物、氘代的技术,应用在医药领域,能够解决毒副作用、耐药性等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
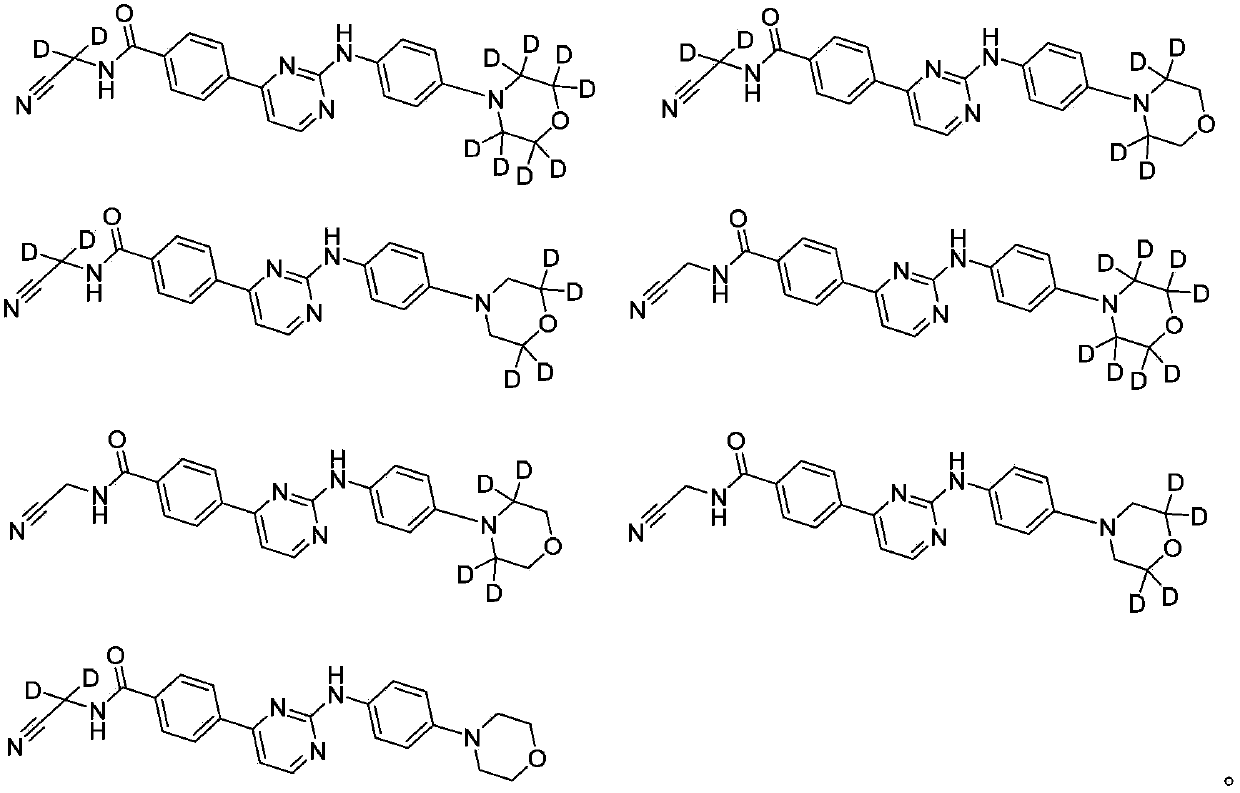
[0103] Embodiment 1: Preparation of N-(cyanomethyl)-4-(2-(4-(d 8 -morpholine)phenylamino)pyrimidin-4-yl)benzamide (compound 9)
[0104]
[0105] 1. Preparation of 4-(4-nitrophenyl)(d 8 -morpholine) (Compound 3)
[0106] In the flask, add compound 4-chloronitrobenzene (3.53g, 22.4mmol), d 8- Morpholine (2.35g, 24.6mmol) and potassium carbonate (6.07g, 44mmol). Dimethylsulfoxide (40 mL) was added. After the addition, the temperature was raised to 100° C. and stirred for 16 hours. TLC detection (ethyl acetate / petroleum ether=1 / 10) showed that the reaction was complete. After cooling to room temperature, water (100 mL) was added to quench the reaction. Dichloromethane (100 mL) extracted twice. The organic layers were combined and washed successively with water and saturated brine. Dry over anhydrous sodium sulfate. Filter and concentrate the filtrate under vacuum with a rotary evaporator to obtain the crude product. Crystallization in a mixed solvent of ethyl acetate ...
Embodiment 2
[0115] Embodiment 2 prepares N-(cyano group (d 2 -Methyl))-4-(2-(4-(d 8 -morpholine)phenylamino)pyrimidin-4-yl)benzamide;
[0116]
[0117] By the method described in Example 1, the difference is: use 2-amino-2,2-d 2 -Acetonitrile hydrochloride is replaced by 2-aminoacetonitrile hydrochloride to obtain the target compound.
Embodiment 3
[0118] Embodiment 3 prepares N-(cyano group (d 2 -Methyl))-4-(2-(4-(2',2',6',6'-d 4 -morpholine)phenylamino)pyrimidin-4-yl)benzamide;
[0119]
[0120] By the method described in Example 1, the difference is: use 2-amino-2,2-d 2 -Acetonitrile hydrochloride replaces 2-aminoacetonitrile hydrochloride, 2,2,6,6-d 4 - Morpholine replaces d 8 -morpholine, thereby obtaining the target compound.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com