Method for preparing icatibant
A preparation process and coupling technology, applied in the field of preparation of polypeptide drugs, can solve the problems of many impurities, unsuitable for industrial scale production, low synthesis yield, etc., and achieve the effect of low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] Embodiment one: the synthesis of Boc-D-Arg-OSu activated ester
[0091] Weigh 310.67g Boc-D-Arg-OH.HCl (1.0mol), add 138.10g HOSu (1.2mol) into 2000ml DMF, add 247.59g DCC (1.2mol) under ice-water bath, react for 1 hour, heat up to room temperature for reaction After 3 hours, the reaction solution was filtered, the mother liquor was spin-dried, dissolved in DCM, filtered, recrystallized from ice ethanol three times, filtered, and dried by a solid oil pump to obtain 273.90 g of Boc-D-Arg-OSu.HCl activated ester with a yield of 89%.
Embodiment 2
[0092] Example 2: Synthesis of Boc-D-Arg-Arg-OH.2HCl
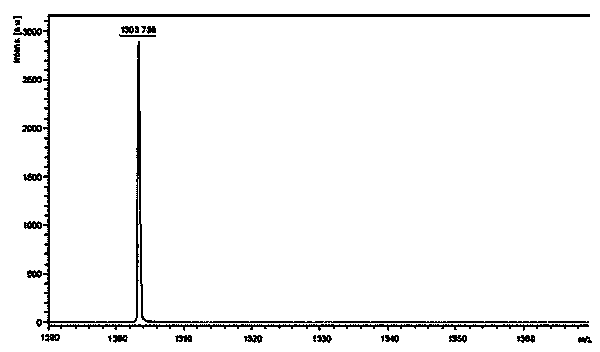
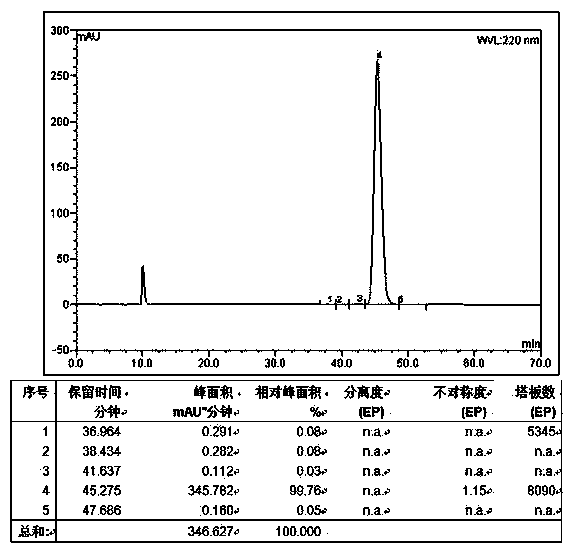
[0093] Weigh 87.10g H-Arg-OH (0.5mol), 153.88g Boc-D-Arg-OSu.HCl (0.5mol) and 79.50g Na 2 CO 3 (0.75mol) was added to a mixed solution of 500ml water and 500ml THF to dissolve, react overnight at room temperature, adjust the pH to 7 with 10% dilute hydrochloric acid, remove THF by rotary evaporation, and then adjust the pH to 3. A large white precipitate was obtained which was filtered. The obtained white precipitate was recrystallized with ice ethanol, and the obtained solid was stirred and recrystallized in dioxane hydrochloride solution for 2 hours. HPLC spectrum such as figure 2 Shown, HPLC purity is 97.95%, yield 87%; Its mass spectrum is as image 3 Shown, [M+Na] + : 453.255, [M+K] +: 469.605, the theoretical precise molecular weight of the dipeptide fragment Boc-D-Arg-Arg-OH.2HCl is: 430.27, the mass spectrometry result of the sample is consistent with the theoretical molecular weight, and the structure is co...
Embodiment 3
[0094] Embodiment three: the degree of substitution is the synthesis of Fmoc-Arg(Pbf)-king resin of 0.60mmol / g
[0095] Weigh 20 g of Wang resin with a degree of substitution of 1.0 mmol / g, add it to a solid-phase reaction column, wash it once with DMF, and after swelling the resin with DMF for 30 minutes, take 64.88 g of Fmoc-Arg (Pbf)-OH (100 mmol) 13.51g HOBt (100mmol) was dissolved in DMF, after adding 12.62g DIC (100mmol) under ice-water bath to activate, add in the reaction column that above-mentioned resin is housed, add 1.22g DMAP (10mmol) after 5 minutes, after reacting for 2 hours, Washed 3 times with DMF, washed 3 times with DCM, capped overnight with 200ml of acetic anhydride and pyridine with a volume ratio of 1:1, shrunk and dried with methanol to obtain Fmoc-Arg(Pbf)-King resin, the detection degree of substitution was 0.60mmol / g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com