Method for modifying diaryl dihydrophenazine through light adjustment.
A technology of diaryldihydrophenazine and light adjustment, which is applied in the direction of organic chemistry, can solve the problems of many reaction steps, low final product yield, low yield, etc., and achieve the effect of high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
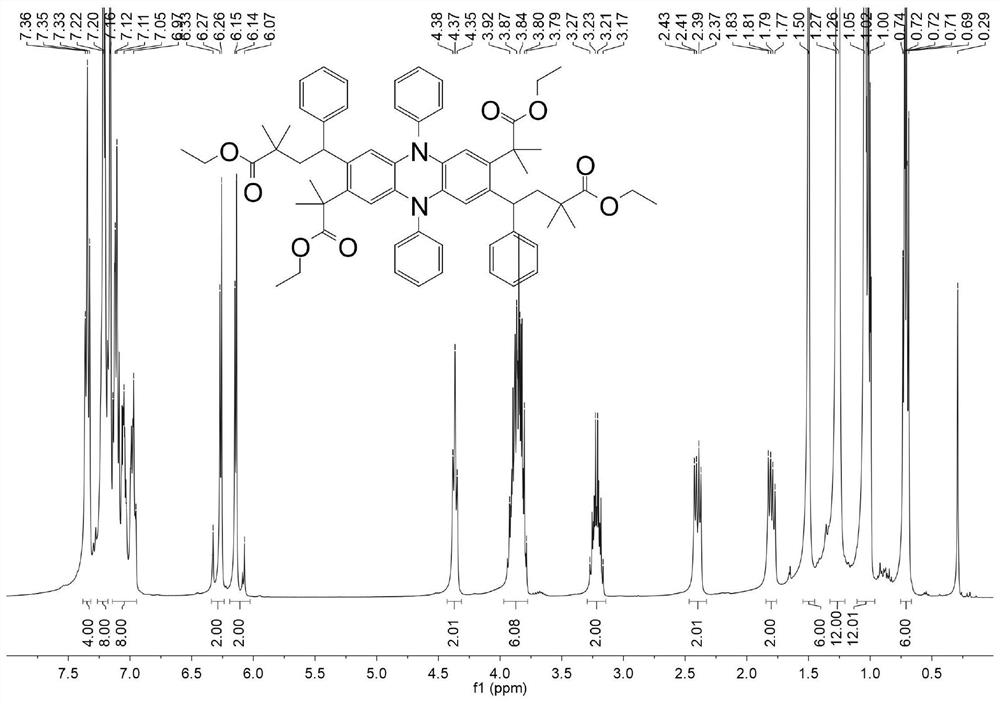
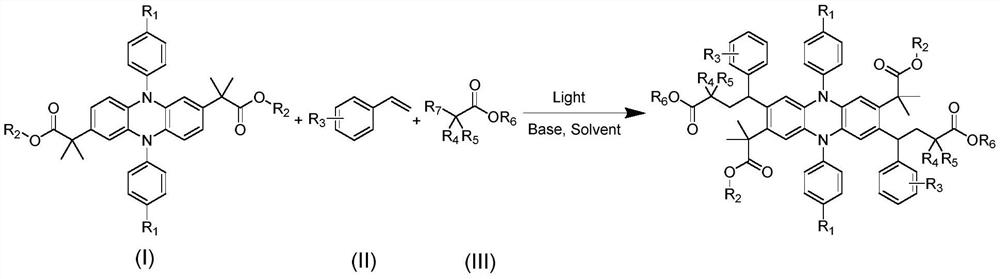
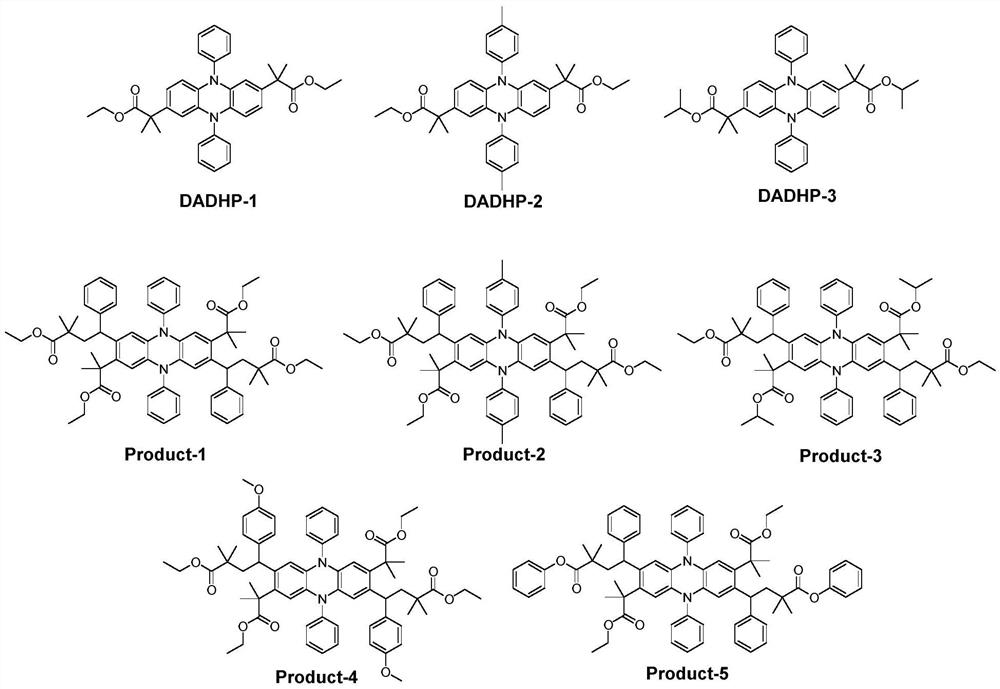
[0028] Example 1: At room temperature, add polytetrafluoroethylene magnetic stirrer, DHDAP-1 (50mg, 0.0889mmol, 1eq), styrene (93mg, 0.8892mmol, 10eq), 2-bromo -Ethyl 2-methylpropionate (347mg, 1.7785mmol, 20eq), sodium carbonate (20mg, 0.1778mmol, 2eq), 1,4-dioxane (5mL), plugged with a rubber stopper and sealed, replaced Air in the bottle and flush with nitrogen. Then the reaction bottle was placed under the condition of light (420-430nm) and reacted for 24 hours. Separation was carried out by column chromatography to obtain the product Product-1 (pale yellow solid, 79 mg, conversion rate 89%).
Embodiment 2
[0029] Example 2: At room temperature, add polytetrafluoroethylene magnetic stirrer, DHDAP-2 (50mg, 0.0847mmol, 1eq), styrene (88mg, 0.847mmol, 10eq), 2-bromo -Ethyl 2-methylpropionate (330mg, 1.694mmol, 20eq), sodium carbonate (18mg, 0.1694mmol, 2eq), 1,4-dioxane (5 mL), plug the rubber stopper and seal it, replace The air in the bottle was vented and flushed with nitrogen. Then the reaction bottle was placed under the condition of light (420-430 nm) and reacted for 24 hours. Separation was carried out by column chromatography to obtain the product Product-2 (pale yellow solid, 75 mg, conversion rate 86%).
Embodiment 3
[0030] Example 3: At room temperature, add polytetrafluoroethylene magnetic stirrer, DHDAP-3 (50mg, 0.0847mmol, 1eq), styrene (88mg, 0.847mmol, 10eq), 2-bromo -Ethyl 2-methylpropionate (330mg, 1.694mmol, 20eq), sodium carbonate (18mg, 0.1694mmol, 2eq), 1,4-dioxane (5 mL), plug the rubber stopper and seal it, replace The air in the bottle was vented and flushed with nitrogen. Then the reaction bottle was placed under the condition of light (420-430 nm) and reacted for 24 hours. Separation was carried out by column chromatography to obtain the product Product-3 (pale yellow solid, 82 mg, conversion rate 94%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com