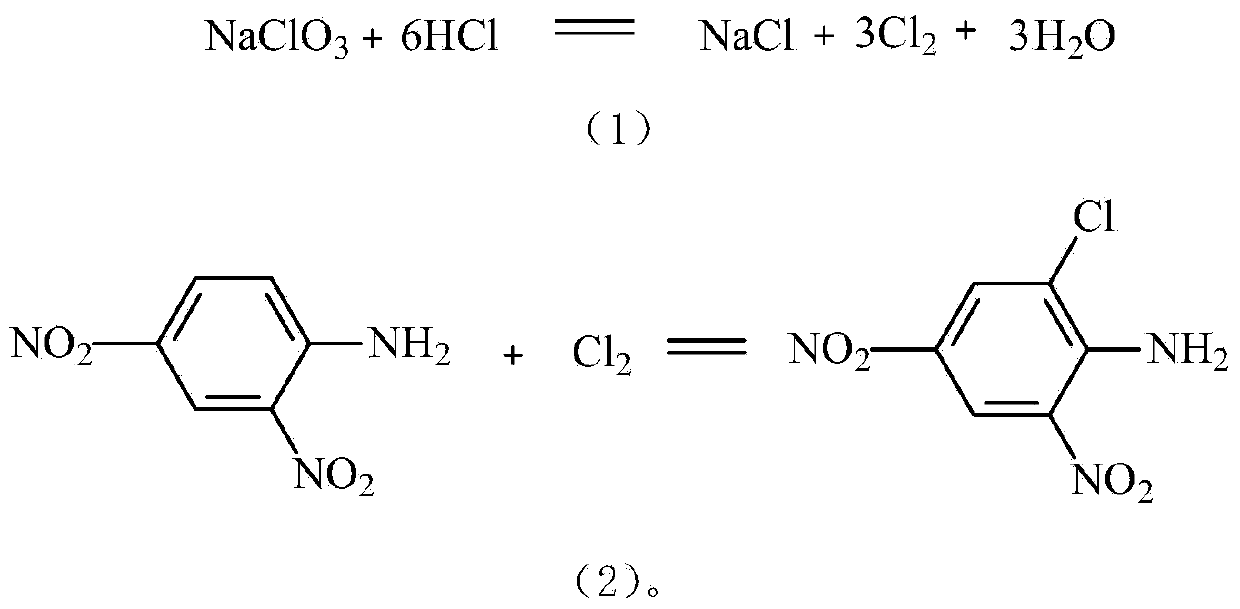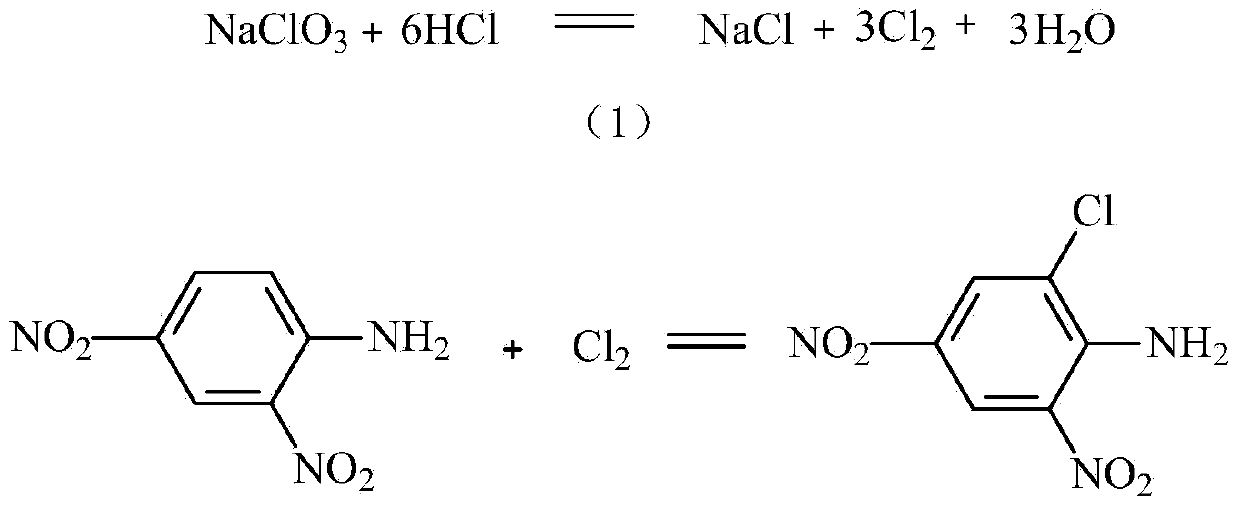Preparation method of 2,4-binitro-6-chloroaniline
A technology of dinitroaniline and chloroaniline, which is applied in the chemical industry, can solve the problems of high local reaction temperature, difficulty in control, and easy explosion of chlorine dioxide, and achieve the effect of improving product quality and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] 611.8kg of 2,4-dinitroaniline, 400kg of water, dispersed to 20±5μm by dispersion pump, added to 1050kg of 30-35% hydrochloric acid, heated to 70°C, stirred at 70-75°C for 1-2 hours, cooled to 50-60°C for use.
[0043] Dissolve 120kg of sodium perchlorate in 250kg of water to make a sodium chlorate solution below 40°C for use.
[0044] 412kg of 2,4-dinitroaniline solution per hour and 74kg of sodium chlorate solution per hour enter the pipeline reactor at the same time. The total residence time in the pipeline reactor is 3-10 seconds. Hours, when the end point is reached, it is centrifuged and dried, air-dried, the mother liquor is treated and recycled, and dried to obtain 710-720kg of 2.4-dinitro-6-chloroaniline with a water content of ≤2% and a yield of about 98%.
Embodiment 2
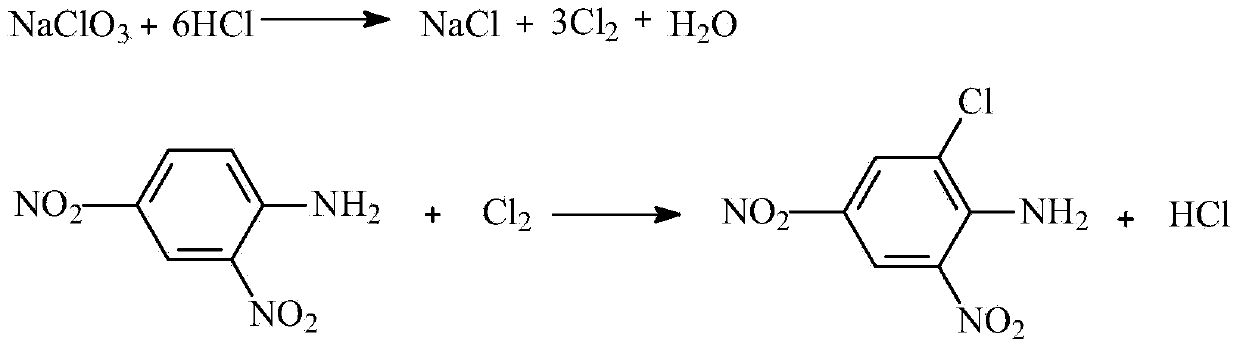
[0046] The weight of 2,4-dinitroaniline is 611.8kg, and the mother liquid water containing 15% hydrochloric acid is 2100kg. After beating evenly, the dispersion pump is dispersed to 20±5μm for use.
[0047] 120kg of sodium perchlorate and 300kg of water are used to make a sodium chlorate solution below 40°C for use.
[0048] Use a metering pump to preheat 500kg of 2,4-dinitroaniline liquid per hour to 50-60°C through the preheater, and enter the pipeline reactor. At the same time, the metering pump injects 84kg of sodium chlorate into the pipeline for reaction In the pipeline reactor, the continuous reaction residence time of the pipeline reactor is 3-10 seconds, enter the aging tank and keep at 70±10°C for 2 hours, central control, centrifuge, and dry to obtain 2.4-dinitro -6-chloroaniline 715kg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com