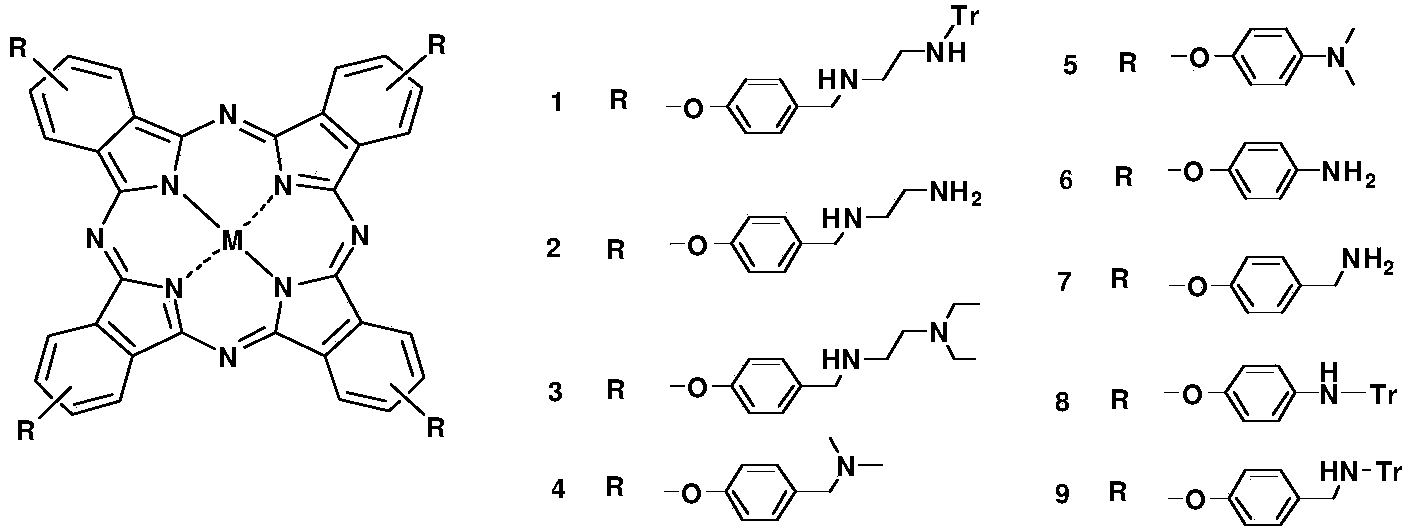
Polyamine phthalocyanine and derivative thereof as well as preparation and application of polyamine phthalocyanine and derivative thereof
A technology of phthalocyanine derivatives and derivatives, applied in medical preparations containing active ingredients, drug combinations, organic chemistry, etc., can solve the problems of lack of phthalocyanine derivatives, low aggregation degree, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
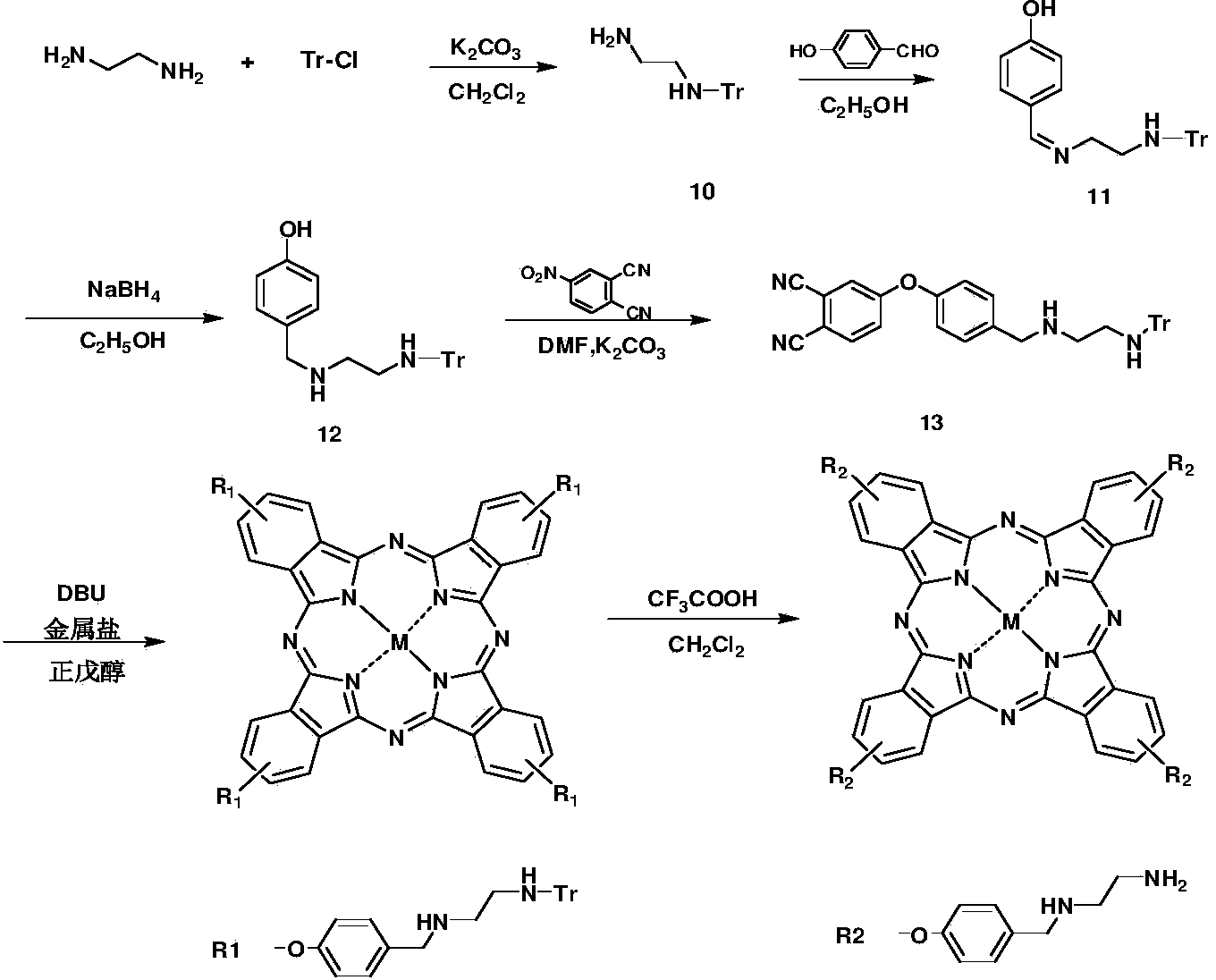
[0075] Polyamine phthalocyanine R1 and preparation method thereof, represented by reaction formula as figure 2 shown.
[0076] (1) Under the protection of nitrogen, the dichloromethane solution of triphenylchloromethane is slowly added dropwise into the dichloromethane solution of ethylenediamine with a constant pressure titration funnel, the molar ratio of triphenylchloromethane and ethylenediamine 1:3, titrated for about 4 hours, then stirred at room temperature for 4 hours to obtain intermediate 10;
[0077] (2) Add intermediate 10 and p-hydroxybenzaldehyde to the ethanol solution at a molar ratio of 1:1.1, add 1.5 equivalents of sodium borohydride after reacting for 3 hours, and continue stirring for 3 hours to obtain intermediate 12;
[0078] (3) Under nitrogen protection, intermediate 12, basic catalyst and 4-nitrophthalonitrile were added to DMF at a molar ratio of 1:2:1.2, and reacted at 40°C for 4 hours to obtain intermediate 13;
[0079] (4) Under the protection of...
Embodiment 2
[0081] Polyamine phthalocyanine R2 and preparation method thereof, represented by reaction formula as figure 2 shown.
[0082] (1) Under the protection of nitrogen, the dichloromethane solution of triphenylchloromethane is slowly added dropwise into the dichloromethane solution of ethylenediamine with a constant pressure titration funnel, the molar ratio of triphenylchloromethane and ethylenediamine 1:3, titrated for about 4 hours, then stirred at room temperature for 4 hours to obtain intermediate 10;
[0083] (2) Add intermediate 10 and p-hydroxybenzaldehyde to the ethanol solution at a molar ratio of 1:1.1, add 1.5 equivalents of sodium borohydride after reacting for 3 hours, and continue stirring for 3 hours to obtain intermediate 12;
[0084] (3) Under nitrogen protection, intermediate 12, basic catalyst and 4-nitrophthalonitrile were added to DMF at a molar ratio of 1:2:1.2, and reacted at 40°C for 4 hours to obtain intermediate 13;
[0085] (4) Under the protection of...
Embodiment 3
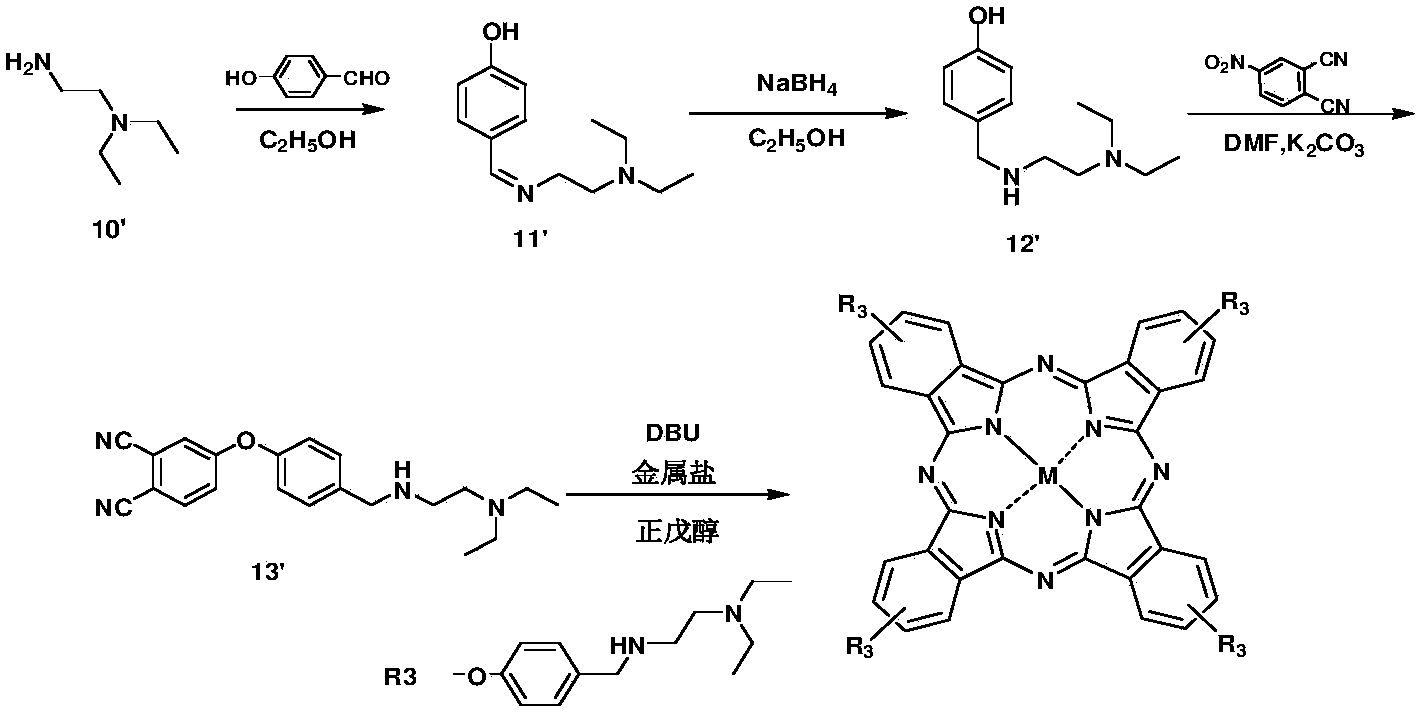
[0088] Polyamine phthalocyanine R3 and its preparation, represented by the reaction formula as image 3 shown.
[0089](1) Diethylethylenediamine 10' and p-hydroxybenzaldehyde were added to the ethanol solution at a molar ratio of 1:1.1, and 1.5 equivalents of sodium borohydride was added after the reaction for 3 hours, and the stirring reaction was continued for 3 hours to obtain the intermediate 12';
[0090] (2) Under nitrogen protection, intermediate 12', basic catalyst and 4-nitrophthalonitrile were added to DMF at a molar ratio of 1:2:1.2, and reacted at 40°C for 4 hours to obtain intermediate 13' ;
[0091] (3) Under the protection of nitrogen, intermediate 13', metal salt, and DBU are added in n-amyl alcohol in a molar ratio of 1:1:1, and the polyamine phthalocyanine R3 is refluxed for 24 hours;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com