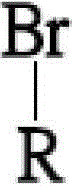Dianhydride monomer and polyimide with side-chain radicals and preparation methods of dianhydride monomer and polyimide
A dianhydride monomer, polyimide technology, applied in the direction of organic chemistry, can solve the problems of poor solubility and reduce the processability of polyimide, and achieve the effect of good solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
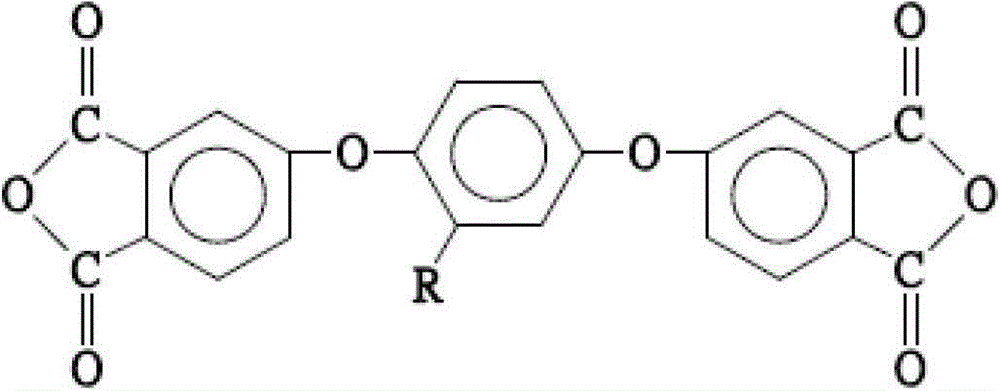
[0040] The dianhydride monomer (e) of the first embodiment of the present invention is 1,4-bis(3,4-dicarboxyphenoxy)-2-adamantylphthalic anhydride, and its structure is as follows:
[0041]
[0042] The preparation method of this dianhydride monomer includes four main steps: forming 2-adamantyl hydroquinone (2-adamantyl hydroquinone), 1,4-bis(3,4-dicyanophenoxy)- 2-adamantylbenzene (1,4-bis(3,4-dicyanophenoxy)-2-adamantyl benzene), 1,4-bis(3,4-dicarboxyphenoxy)-2-adamantylbenzene ( 1,4-bis(3,4-dicarboxyphenoxy)-2-adamantyl benzene) and 1,4-bis(3,4-dicarboxyphenoxy)-2-adamantylphthalic anhydride (1,4-bis (3,4-dicarboxyphenoxy)-2-adamantyl benzene dianhydride).
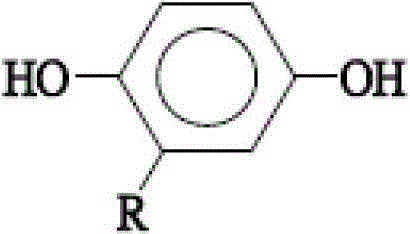
[0043] The step of forming 2-adamantyl hydroquinone comprises, at first 15 grams (69.77mmol) of 1-bromoadamantane, 15.35 grams (139.5mmol) of hydroquinone and 75 milliliters of benzene are added into 250 milliliters of there-necked flasks with nitrogen gas In, heating to reflux for 72 hours, the reaction temperatur...
Embodiment 2
[0067] The polyimide compound of the second embodiment of the present invention uses 1,4-bis(3,4-dicarboxyphenoxy)-2-adamantylphthalic anhydride as the dianhydride monomer (e) of the present invention, And take the III group as the main chain of the diamine monomer (f III). It is worth noting that the group III is used as the group of the diamine monomer here, so the compound with the III group is, for example, a diamine monomer (f III), a polyamic acid compound (gIII) and a polyimide (h III), III will be marked after the lowercase English letter mark. The structure of the polyimide (h III) of the second embodiment of the present invention is as follows:
[0068]
[0069] In addition, the preparation method of the polyamic acid compound (g III), that is, the method of thermal cyclization, has been described in detail above, so it will not be repeated here.
[0070] Next, we will introduce the preparation of polyimide compounds by chemical cyclization. This preparation inc...
Embodiment 3
[0077] The polyimide compound of the third embodiment of the present invention uses 1,4-bis(3,4-dicarboxyphenoxy)-2-adamantylphthalic anhydride as the dianhydride monomer (e) of the present invention, And the I group is used as the main chain of the diamine monomer (f I). And its structure of the polyimide (h I) of the third embodiment of the present invention is as follows:
[0078]
[0079] It is worth mentioning that the preparation method of polyimide (h I), that is, chemical cyclization, has been described in detail above, so it will not be repeated here. In addition, the I group is used as the group of the diamine monomer here, so the compound with the I group is, for example, a diamine monomer (f), a polyamic acid compound (g) and a polyimide compound (h ), and I will be marked behind the lowercase English letter mark to make a distinction. The subsequent II groups and IV-VIII groups can be deduced in the same way, and will not be described in detail later.
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com