Kit for treating retinal disease
A technology for retinal diseases and kits, applied in the field of medical molecular biology, can solve problems such as retinal infection, hindering the dedifferentiation and proliferation of Müller cells, and the short-term effect of exogenous nutritional factors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1, Preparation of recombinant adenovirus overexpressing RNA-binding protein Lin28B
[0057] Step (1) Preparation of Lin28B-encoding gene
[0058] Human Lin28B coding gene sequence: (GenBank: DQ127228.1)
[0059] Rat Lin28B coding gene sequence: (NCBI Reference Sequence: XM_001069344.2)
[0060] Step (2) Prepare the adenovirus shuttle vector pAV-MCMV-Lin28B-GFP-3FLAG
[0061] Include the following main steps:
[0062] The target gene was amplified by PCR using the chemically synthesized LIN28B gene as a template.
[0063] After digestion of the expression vector, the vector fragment was recovered by gel.
[0064] Transform E.coli competent cells after homologous recombination between the target gene and the vector fragment.
[0065] The transformants were identified by colony PCR, the positive clones were sent for sequencing, and the sequence verification was correct as the adenovirus shuttle vector pAV-MCMV-Lin28B-GFP-3FLAG. Clones were subjected to plasmi...
Embodiment 2
[0186] Embodiment 2, the formula of buffer solution and preparation method
[0187] The formula of the buffer solution is: 3% sucrose is dissolved in 1×PBS, that is, 3 g of sucrose is weighed and dissolved in 100 ml of 1×PBS, and sterilized by filtering through a 0.2 μm filter.
[0188] The preparation method of 1×PBS is as follows: 8g NaCl, 0.2g KCl, 1.44g Na2HPO4, 0.24g KH2PO4 are dissolved in 1 liter of distilled water, the pH value is 7.4, and autoclaved.
Embodiment 3
[0189] Embodiment 3, in vitro experiment:
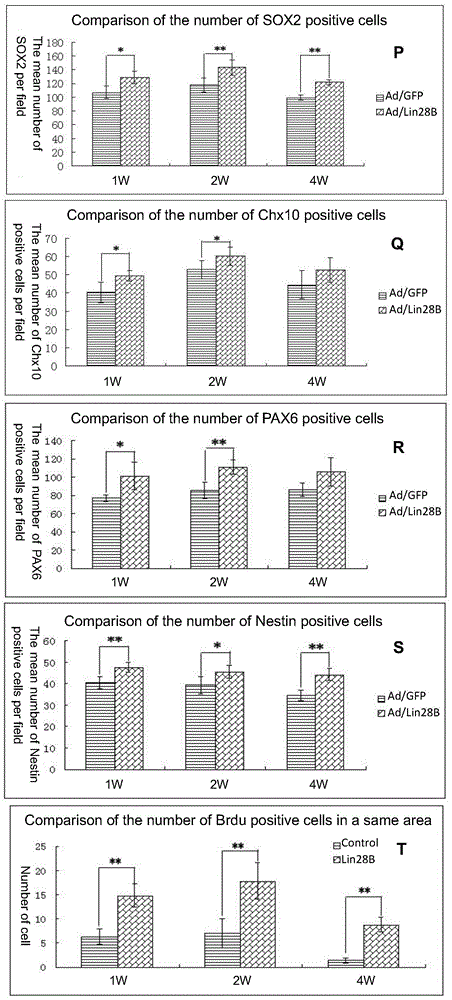
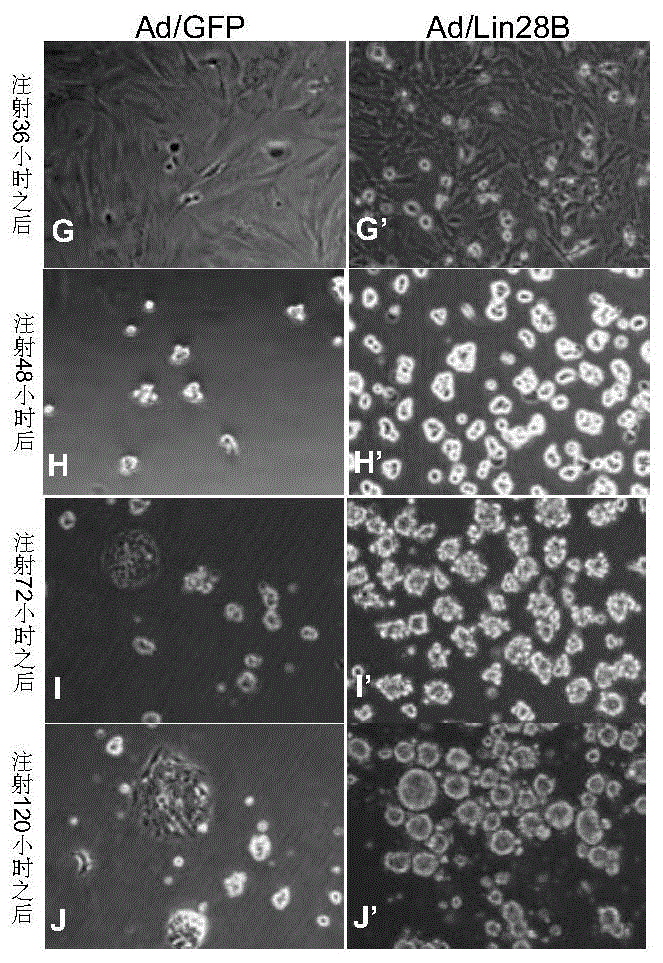
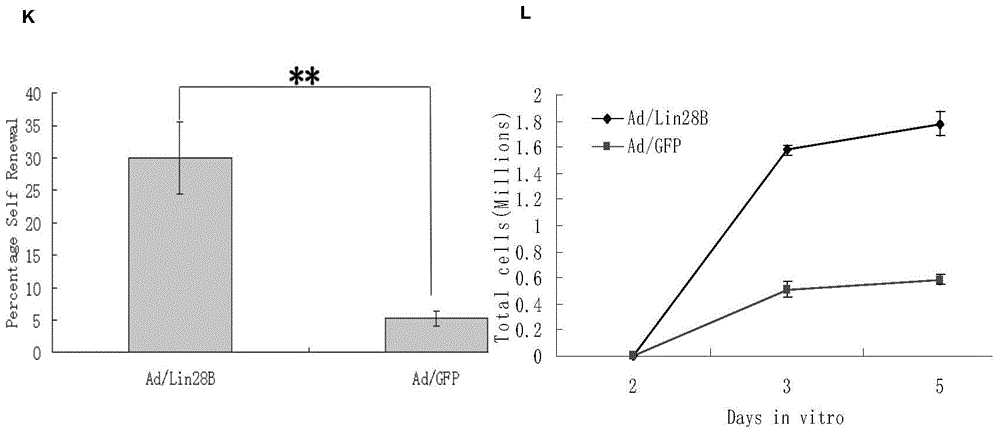
[0190] 1. In vitro observation of Ad / Lin28B on the self-renewal of Müller cells
[0191] 1) Prepare Müller cells infected with Ad / Lin28B.
[0192] Primary Müller Cell Culture Method
[0193] 1. Take a rat (LE mouse species, 7-10 days after birth, 8 days is the best) and place both eyes in Muller cell culture medium overnight (the time should not be too long, and should be controlled within 12 hours).
[0194] 2. Take out the overnight eyeball tissue and put it in the digestion solution (collagenase 0.5+trypsin 0.25) in a 37-degree incubator for 1 hour, and the muller medium stops the digestion.
[0195] 3. Peel off the retinal tissue on ice under a microscope, wash it once with medium at 4 degrees, and transfer it into an EP tube.
[0196] 4. Add muller cell culture medium into the EP tube and pipette not less than 100 times, gently and with few bubbles.
[0197] 5. After the pipetting is completed, balance it into the centrifuge...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com