Five-membered heterocyclic derivative-bridged perylene diimide dipolymer and its preparation method and use in organic photovoltaic device
A perylene diimide dimer and dimer technology, which is applied in the field of asymmetric perylene diimide dimers, can solve the problems of poor solubility and film-forming properties, and limit the application of photovoltaic materials.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] Example 1. Preparation of unsymmetrically substituted 1-bromo-7 alkoxy perylene diimide (compound 4a)
[0105]
[0106] Dissolve 3,4,9,10-perylene diacid anhydride (3.82g, 10mmol, compound 1) in 100mL of pyridine, add 2-ethylhexylamine (6.46g, 50mmol), and heat at 120°C , stirred for 24 hours. After the reaction was completed, the reaction solution was poured into an aqueous solution of 1 mol / L hydrochloric acid (1 L), the precipitate was collected by filtration, and dried under heating at 60° C. to obtain product 2. The product was dissolved in 100 mL of dichloromethane without any treatment, and 20 mL of elemental bromine was added dropwise under heating and reflux conditions. After the addition was complete, heating and reflux was continued overnight. The product 3 was obtained by column with H60 silica gel.
[0107] The product 3 (773 mg, 1.0 mmol, compound 3) was dissolved in 10 mL of DMF, and methoxyethanol (384.5 mg, 5.0 mmol) and K 2 CO 3 (691.1mg, 5.0mmo...
Embodiment 2
[0111] Example 2. Preparation of asymmetrically substituted perylene diimide dimer (compound 5)
[0112]
[0113] The product 3 (773.0 mg, 1.0 mmol) was dissolved in 15 mL of DMF, n-butanol (370.6 mg, 5 mmol) and K 2 CO 3 (691.1mg, 5mmol), stirred and reacted for 1 hour under heating at 80°C. After the reaction was completed, the reaction solution was poured into 50 mL of water, the precipitate was collected by filtration, the calcium precipitate was dissolved in a mixed solvent of 50 mL of dichloromethane and 50 mL of water, the dichloromethane layer was collected by liquid separation, and the dichloromethane was distilled off under reduced pressure to obtain purple A black solid was passed through a column with H60 silica gel to obtain the product 4b.
[0114] The characterization data of this product are as follows:
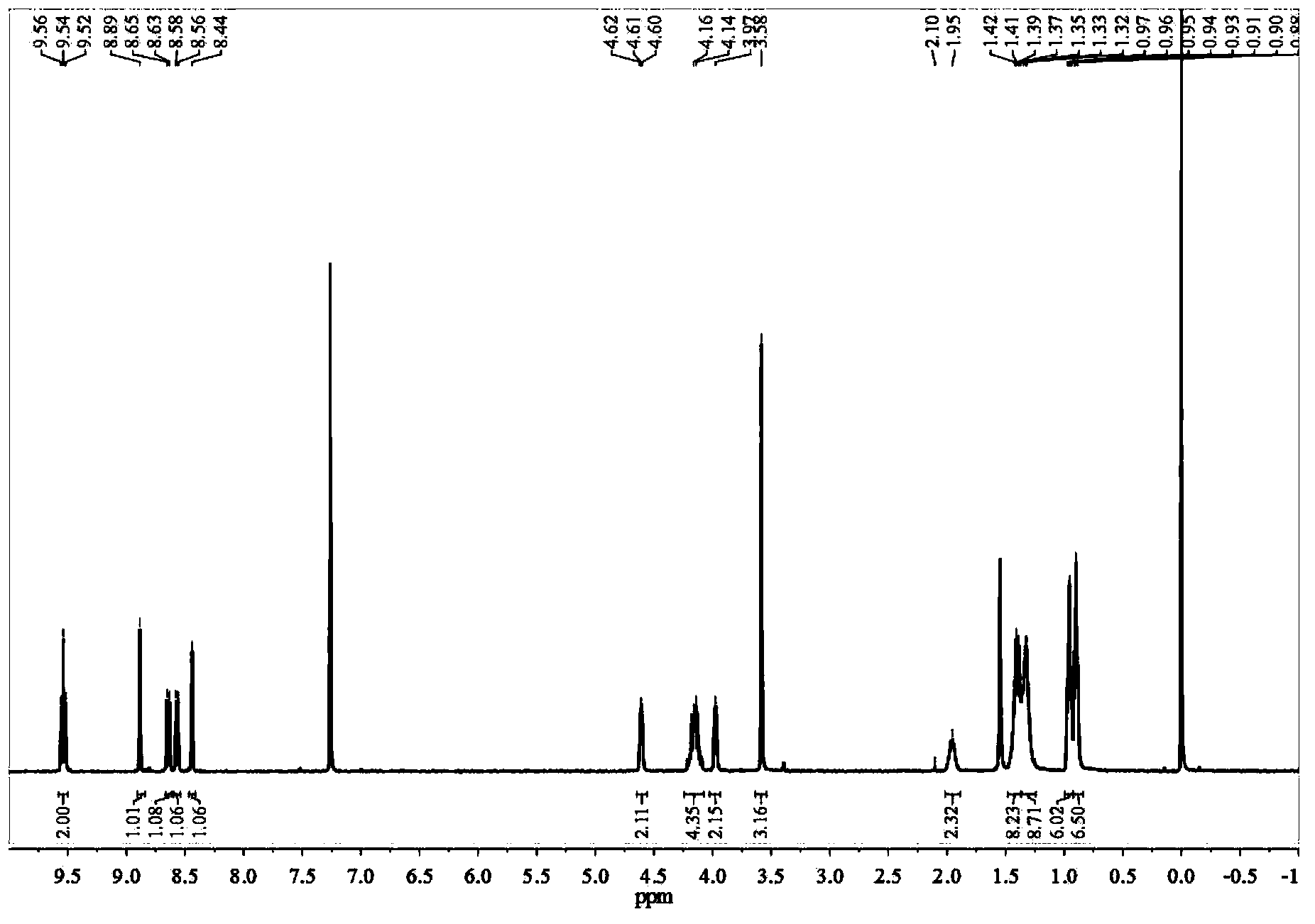
[0115] 1 H-NMR (400MHz, CDCl 3 )δppm:9.47(d,J=8.00Hz,1H),9.34(d,J=8.00Hz,1H),8.79(s,1H),8.58(d,J=8.00Hz,1H),8.50(d, J=8.00Hz,1H),8.39(s,1H),4.46(m,2H...
Embodiment 3
[0119] Example 3. Preparation of asymmetrically substituted perylene diimide dimer (compound 6a)
[0120]
[0121] The product 4a (844.6 mg, 1.1 mmol) was dissolved in 15 mL of toluene, and commercially available thiophene-2,5-di-tributyltin (332.1 mg, 0.5 mmol) and tetrakis(triphenylphosphine) palladium (34.7 mg , 0.03mmol), under the heating condition of 120°C, the reaction was stirred for 36 hours. The toluene in the reaction system was distilled off under reduced pressure to obtain a red-black solid, which was dissolved in a mixed solvent of 50 mL of dichloromethane and 50 mL of water, and the dichloromethane layer was collected by liquid separation, and dichloromethane was distilled off under reduced pressure to obtain a red-black solid. A black solid was passed through a column with H60 silica gel to obtain the product 6a.
[0122] The structural characterization data of this product are as follows:
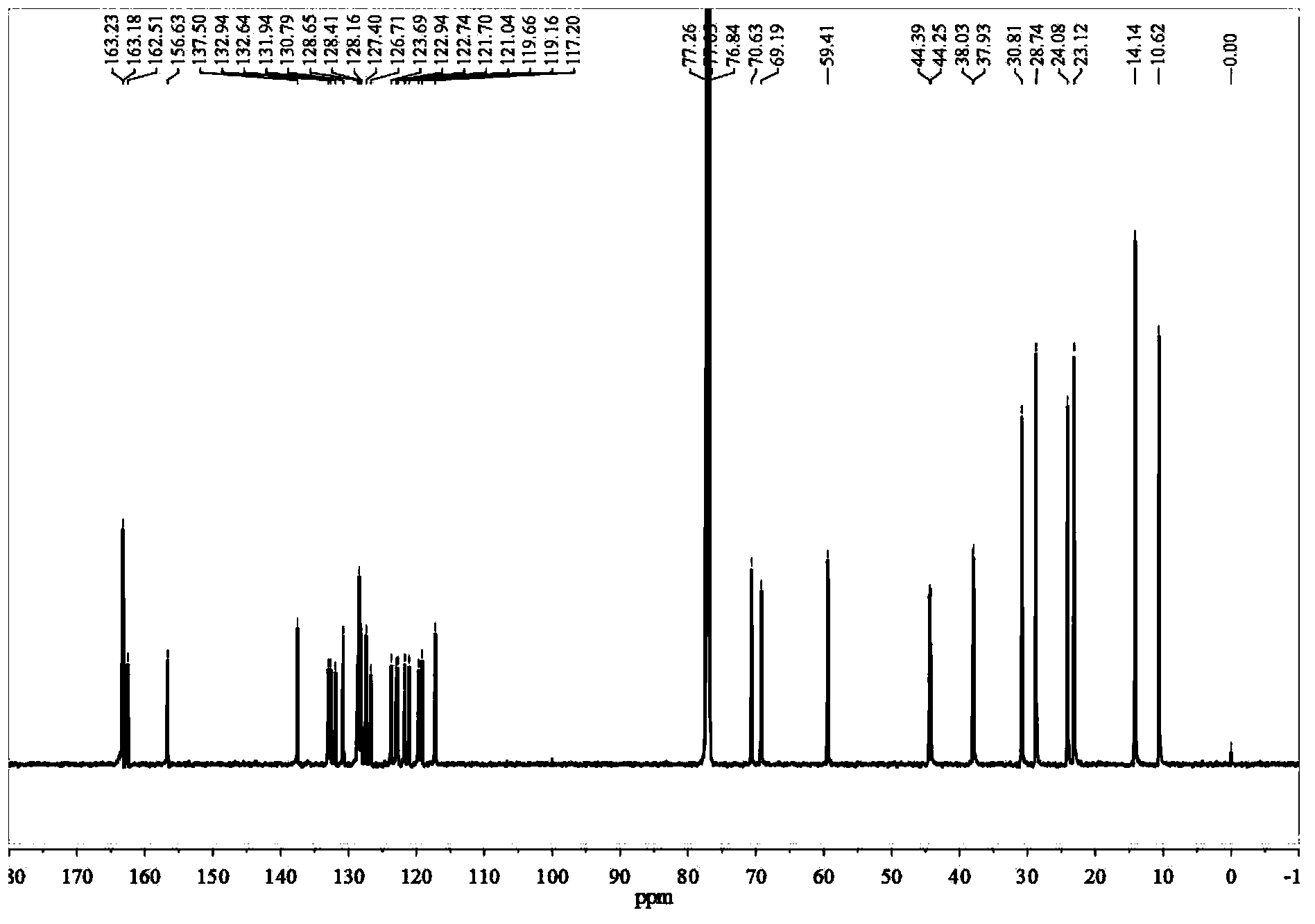
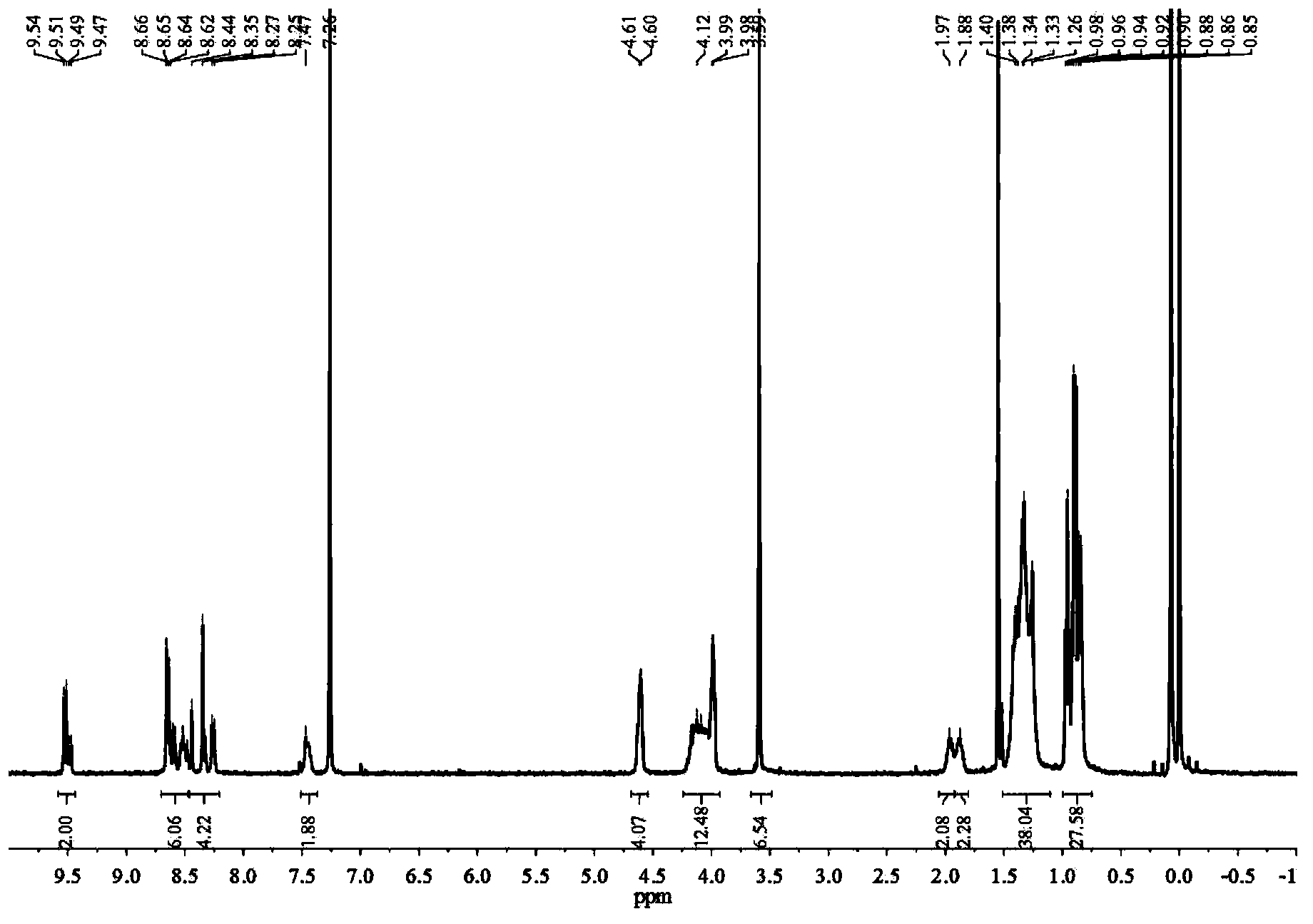
[0123] 1 H-NMR (400MHz, CDCl 3 )δppm:9.53(d,J=8.00Hz,1.40H),9.4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Short circuit current | aaaaa | aaaaa |
| Open circuit voltage | aaaaa | aaaaa |
| Short circuit current | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com