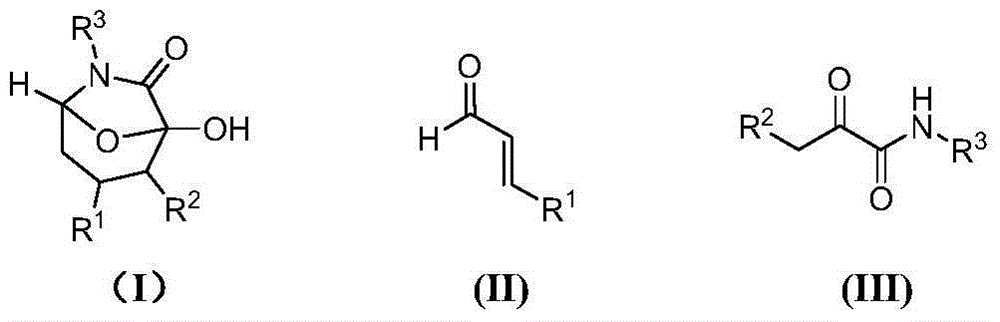
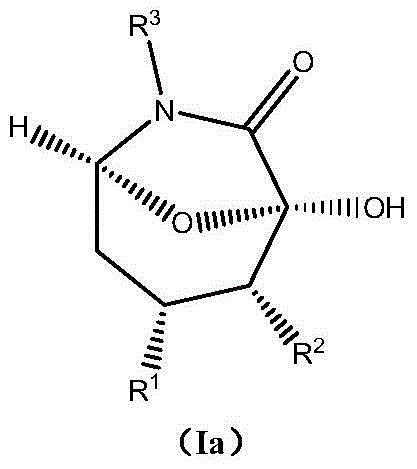
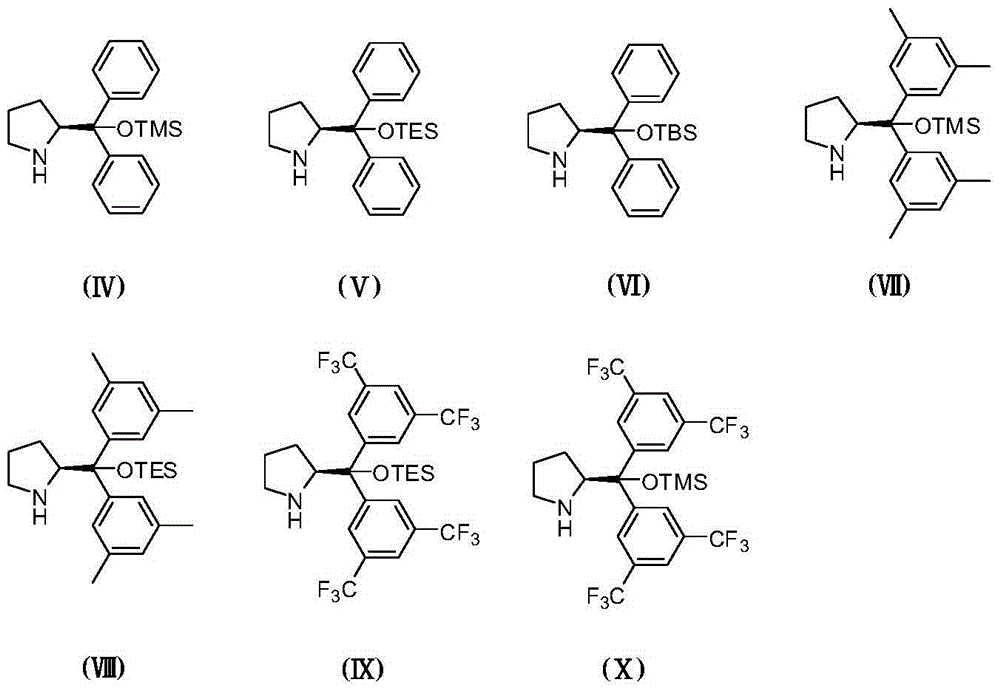
Asymmetric synthesis method of chiral bicyclo-caprolactam compound
A technology of caprolactam and synthesis method, which is applied in the direction of organic chemistry, can solve the problems of environmental pollution, limited application range, difficult removal of metals, etc., and achieve the effect of increasing product yield and reducing purification steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1: (1R, 2R, 3R, 5S)-1-hydroxy-2-methyl-3,6-diphenyl-8-oxo-6-azabicyclo[3,2,1]octane Preparation of -7-one
[0049] Add 2-carbonyl-N-phenylbutyramide (0.0354g, 0.2mmol), cinnamaldehyde (0.0328g, 0.24mmol) and 0.5mL toluene into a 10mL test tube, and add the chiral secondary amine catalyst (VI) (0.017g, 0.04 The reaction was carried out at 0°C for 48h under the co-catalyzed reaction of triethylamine (0.004g, 0.04mmol) and triethylamine (0.004g, 0.04mmol). After extraction with ethyl acetate (3×2mL), the extract was distilled to remove the solvent, and the resulting concentrate was used 200-300 mesh silica gel Column chromatography was performed. The eluent was a mixture of ethyl acetate and petroleum ether in a volume ratio of 1:3. The eluent containing the target compound was collected, concentrated and dried to obtain the target compound (0.0494g, yield 80%, Ee value 99%, 98%, dr value 5.7:1), 1 H NMR(500MHz, CDCl 3 ): δ=7.596-7.580(m,2H),7.396-7.364(m,2H),7.334-7...
Embodiment 2
[0050] Example 2: (1R,2R,3R,5S)-1-hydroxy-2-methyl-3,6-diphenyl-8-oxo-6-azabicyclo[3,2,1]octane Preparation of -7-one
[0051] Add 2-carbonyl-N-phenylbutanamide (0.0354g, 0.2mmol), cinnamaldehyde (0.0328g, 0.24mmol) and 0.5mL toluene into a 10mL test tube, and add the chiral secondary amine catalyst (IV) (0.013g, 0.04 The reaction was carried out at 25°C for 24h under the co-catalyzed reaction of triethylamine (0.004g, 0.04mmol) and triethylamine (0.004g, 0.04mmol). After extraction with ethyl acetate (3×2mL), the extract was distilled to remove the solvent. Column chromatography was performed. The eluent was a mixture of ethyl acetate and petroleum ether in a volume ratio of 1:3. The eluent containing the target compound was collected, concentrated and dried to obtain the target compound (0.0488g, yield 79%, Ee value It is 94%, 97%, dr value 2.8:1).
Embodiment 3
[0052] Example 3: (1R,2R,3R,5S)-1-hydroxy-2-methyl-3,6-diphenyl-8-oxo-6-azabicyclo[3,2,1]octane Preparation of -7-one
[0053] Add 2-carbonyl-N-phenylbutanamide (0.0354g, 0.2mmol), cinnamaldehyde (0.0328g, 0.24mmol) and 0.5mL toluene into a 10mL test tube, and add the chiral secondary amine catalyst (IV) (0.015g, 0.04 The reaction was carried out at 25°C for 24h under the co-catalyzed reaction of triethylamine (0.004g, 0.04mmol) and triethylamine (0.004g, 0.04mmol). After extraction with ethyl acetate (3×2mL), the extract was distilled to remove the solvent. Column chromatography was performed. The eluent was a mixture of ethyl acetate and petroleum ether in a volume ratio of 1:3. The eluent containing the target compound was collected, concentrated and dried to obtain the target compound (0.050g, yield 81%, Ee value It is 95%, 95%, dr value 2.5:1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com