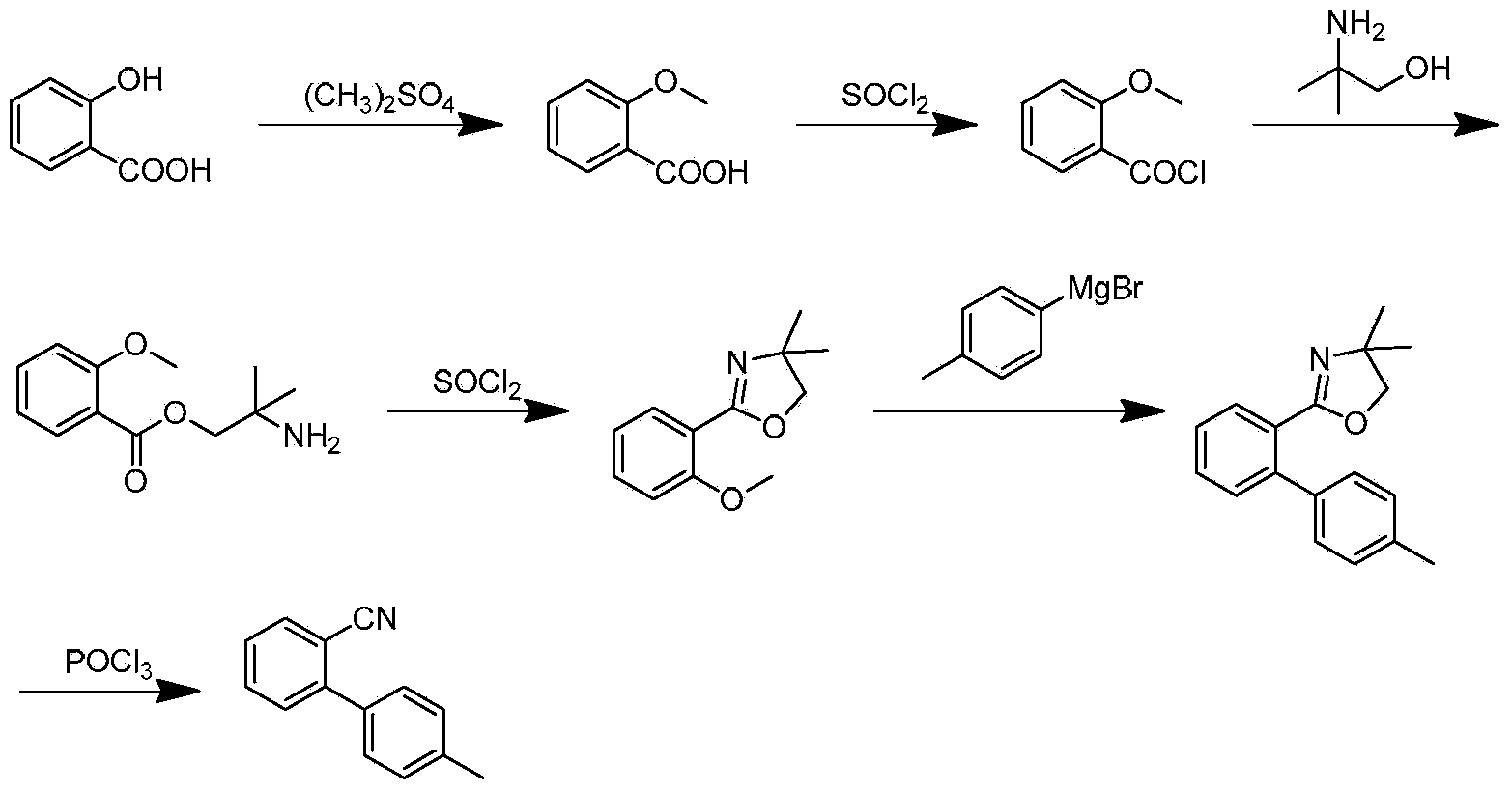
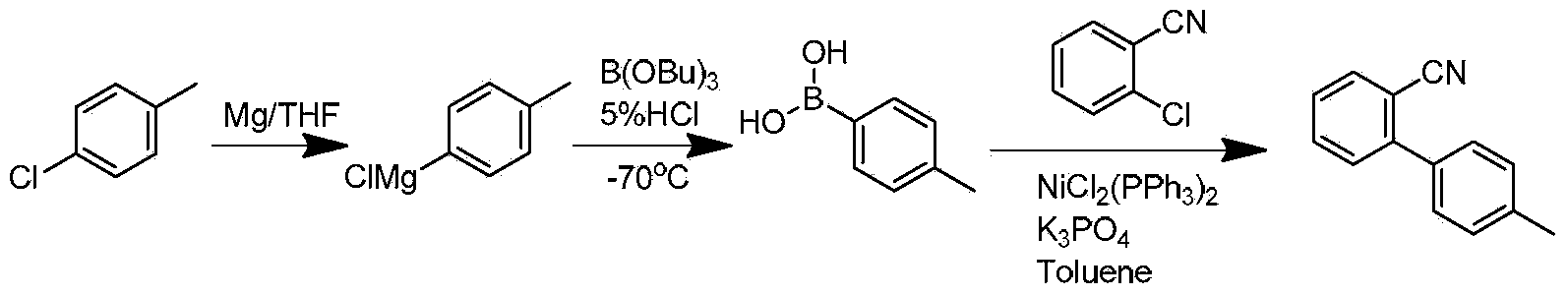
Preparation method for 2- cyanogroup-4 '-methyl diphenyl
A technology of methyl biphenyl and cyano, which is applied in the preparation of 2-cyano-4'-methyl biphenyl and the preparation of antihypertensive drug intermediates, which can solve the problem of high catalyst price and lack of cost advantages. problems, to achieve the effect of enhancing catalytic efficiency, increasing catalyst activity, and improving reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
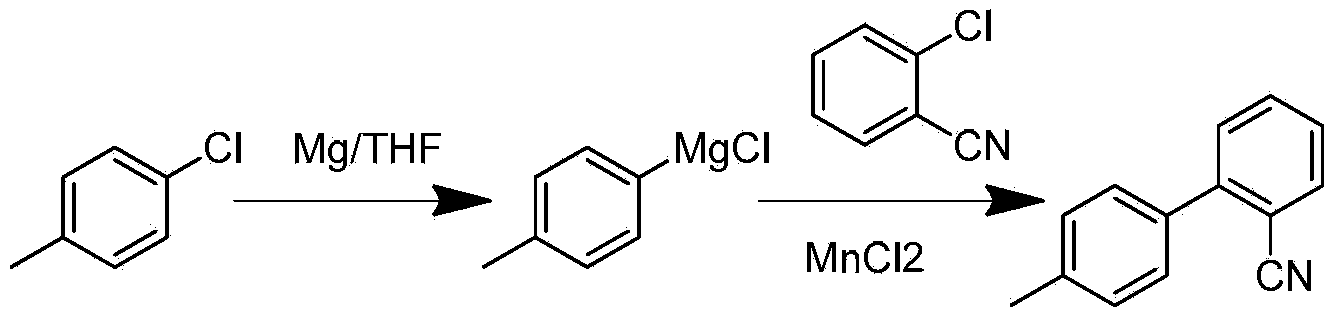
[0041] 1. Preparation of Compound (I) Grignard Reagent
[0042]
[0043] 506g of p-chlorotoluene and 500g of tetrahydrofuran are formulated into p-chlorotoluene solution, 96g of magnesium chips are added in the reaction flask, then 100g of the above-mentioned p-chlorotoluene solution is added, and 4g of initiator (1,2-dibromoethane) is added, Stir and heat up to 60-75°C. After a large number of bubbles are generated and the temperature rises significantly, slowly add the remaining p-chlorotoluene solution dropwise. During the dropping process, keep the temperature at 60-75°C. Qualified, add tetrahydrofuran 1500g to dilute the Grignard solution.
[0044] Two, the preparation of compound (Ⅱ)
[0045]
[0046] Preparation of application solution: 12g MnCl 2 Put it into the reaction bottle, add about 1500g of tetrahydrofuran to the reaction bottle in advance, stir and cool down to -5~5°C. Add 450g o-chlorobenzonitrile and 500g tetrahydrofuran mixed solution. Control the ...
Embodiment 2
[0051] 1. Preparation of compound (I) Grignard solution
[0052]
[0053] Add 96Kg of magnesium chips into the dry Grignard kettle, and at the same time, mix 506Kg of p-chlorotoluene and 500Kg of tetrahydrofuran evenly, and put them into the high-level tank. Dibromoethane (only for batch use), stir and heat up to 60-75°C, after a large number of bubbles appear and the temperature rises significantly, then slowly add the remaining p-chlorotoluene solution dropwise, keep the temperature at 60-75°C during the dropwise addition, add After completion, react at constant temperature for 4 hours. After the sampling test is qualified, 1500Kg of tetrahydrofuran is added to dilute the Grignard solution, and the temperature is lowered to 40-50°C for discharge. If the reaction does not meet the quality standard, the time can be extended or an appropriate amount of magnesium powder can be added.
[0054] Two, the preparation of compound (Ⅱ)
[0055]
[0056] Put 12Kg of catalyst man...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com