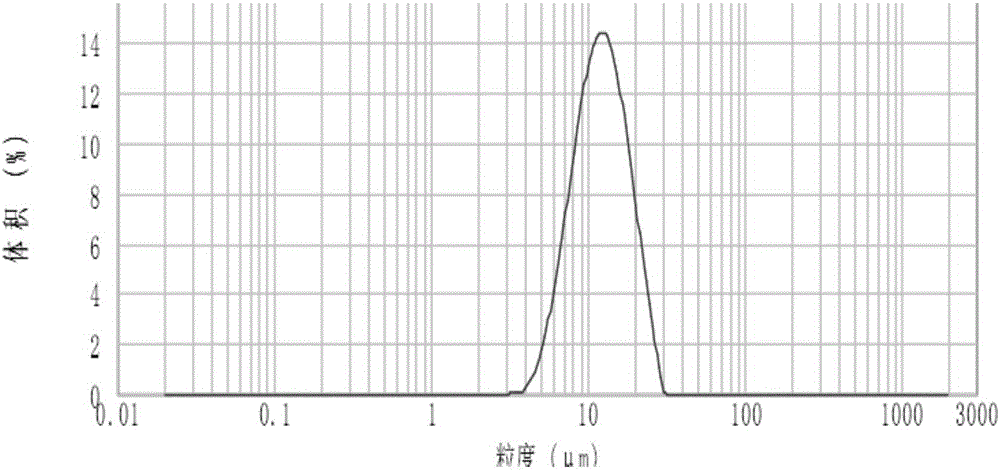
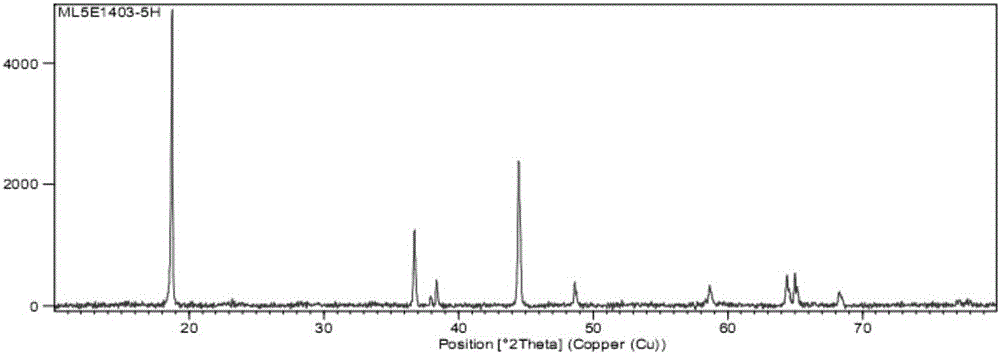
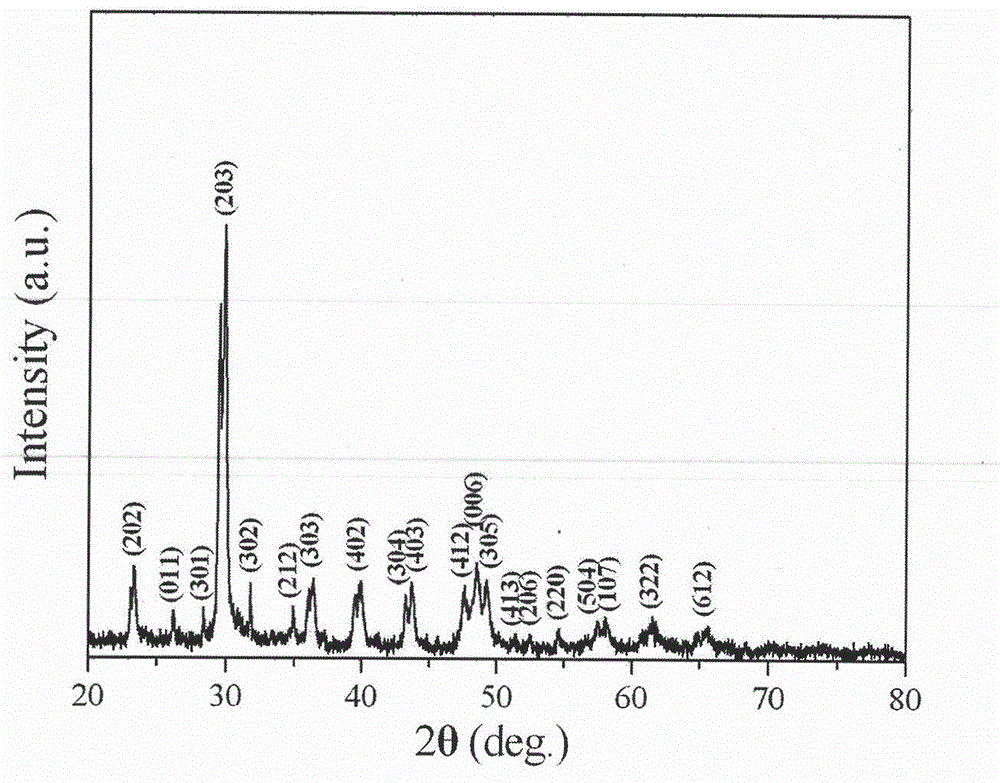
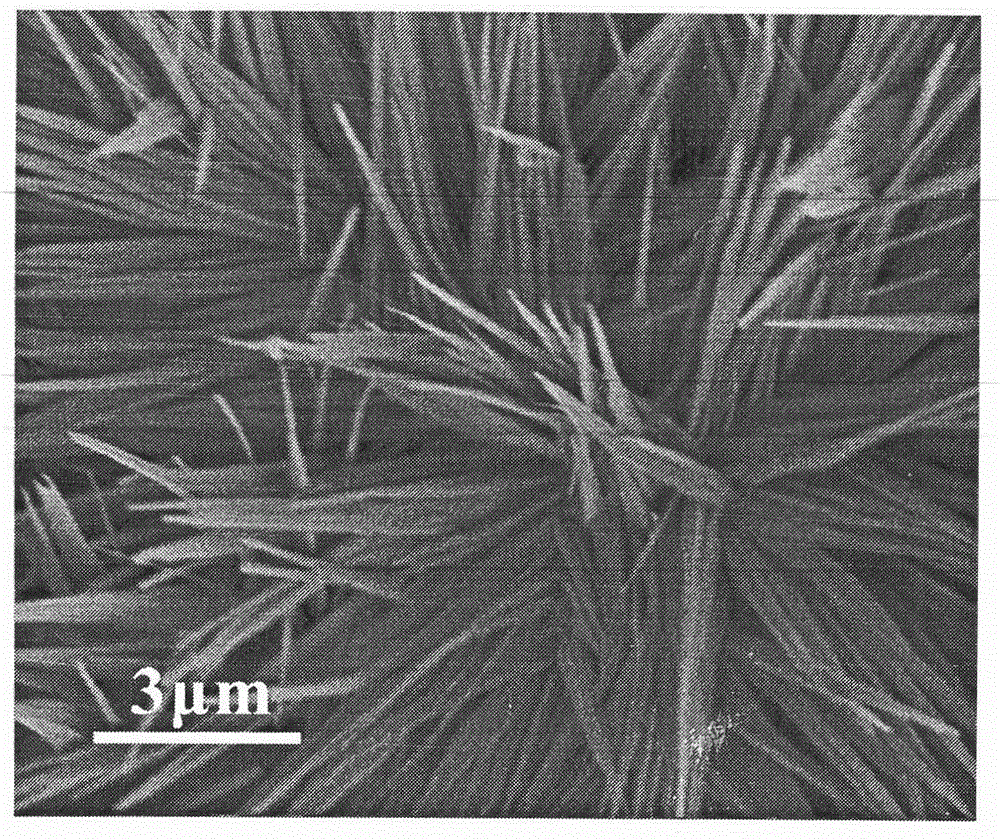
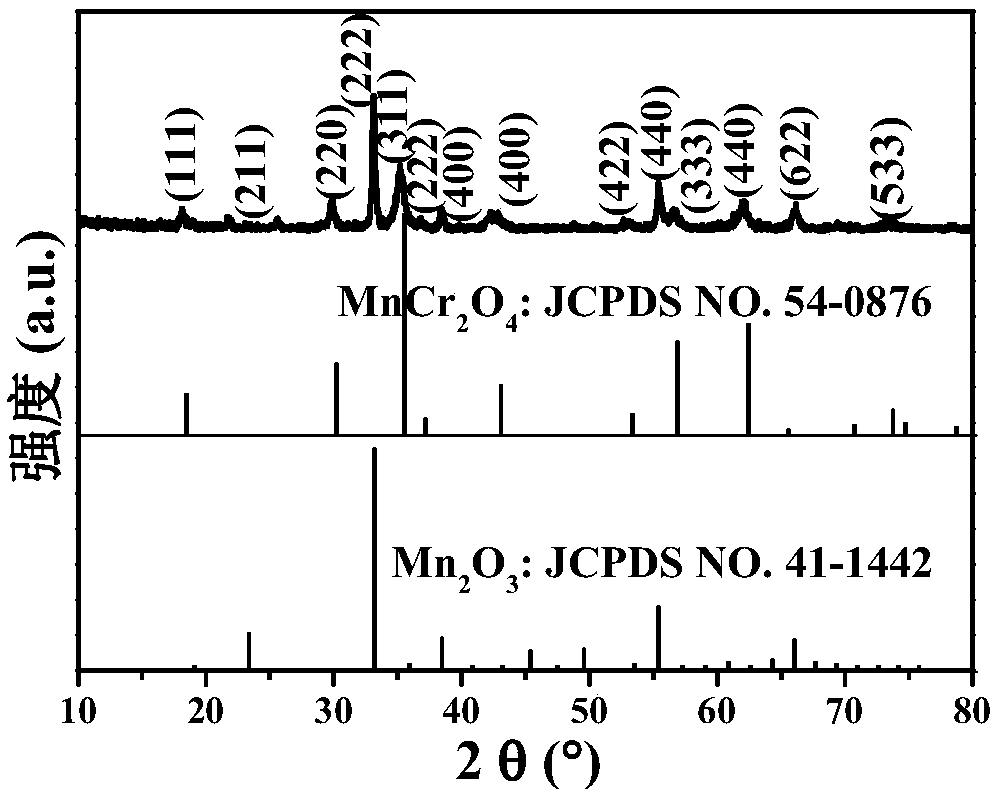
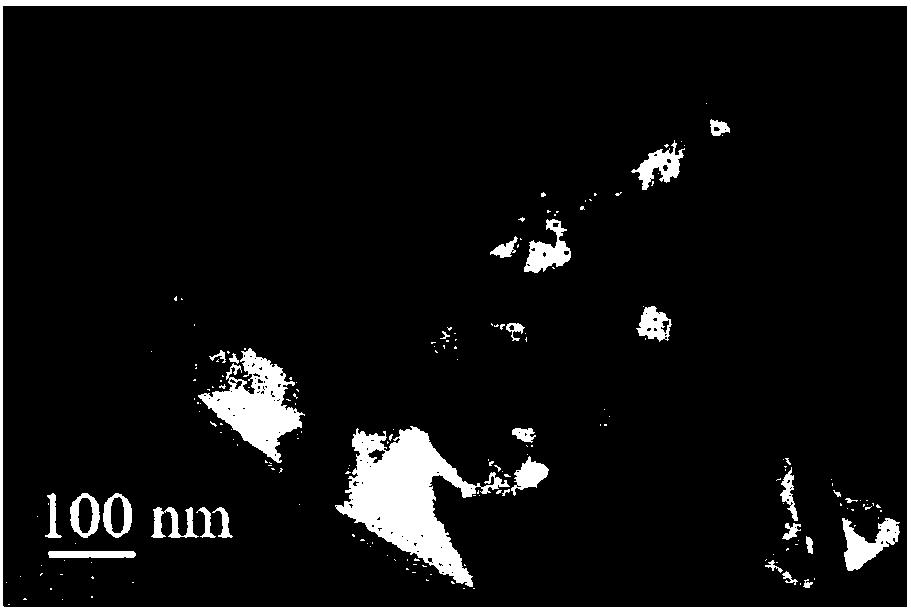
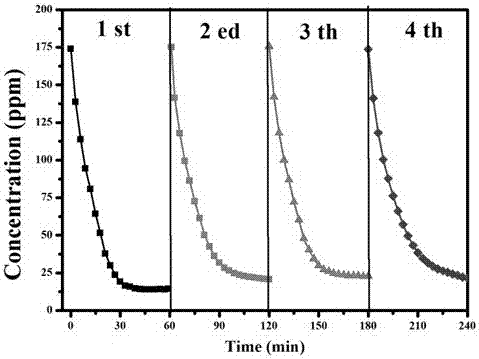
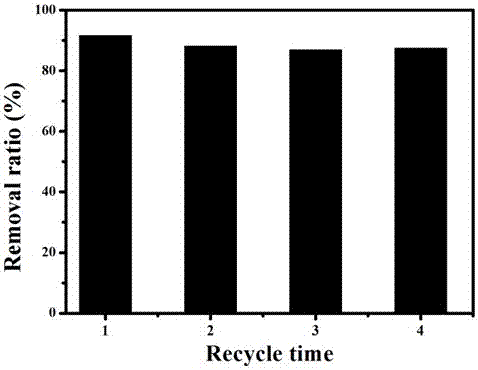
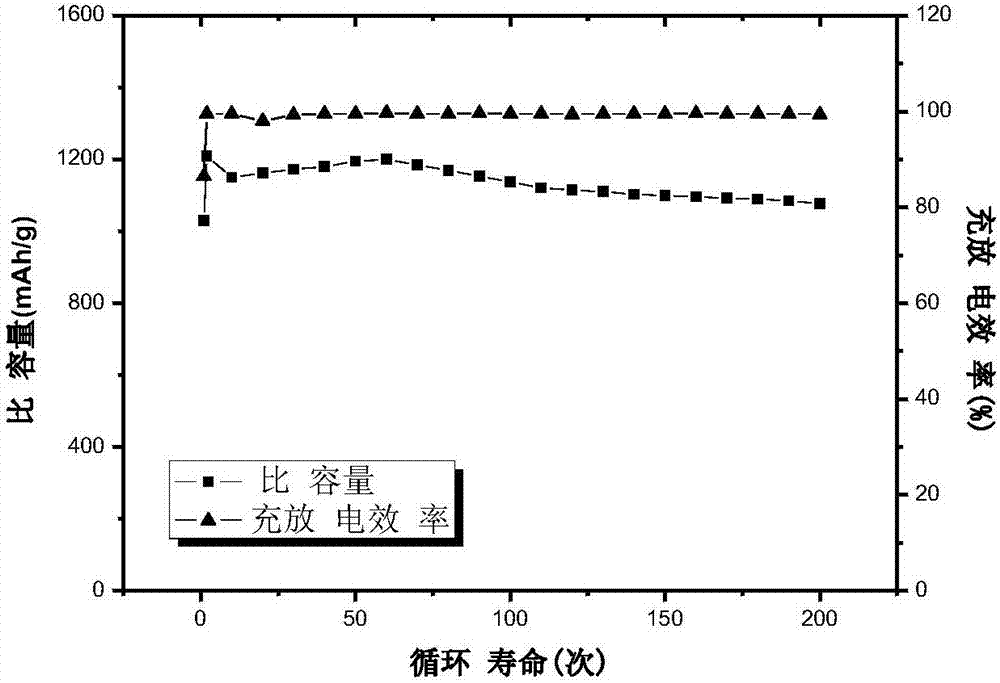
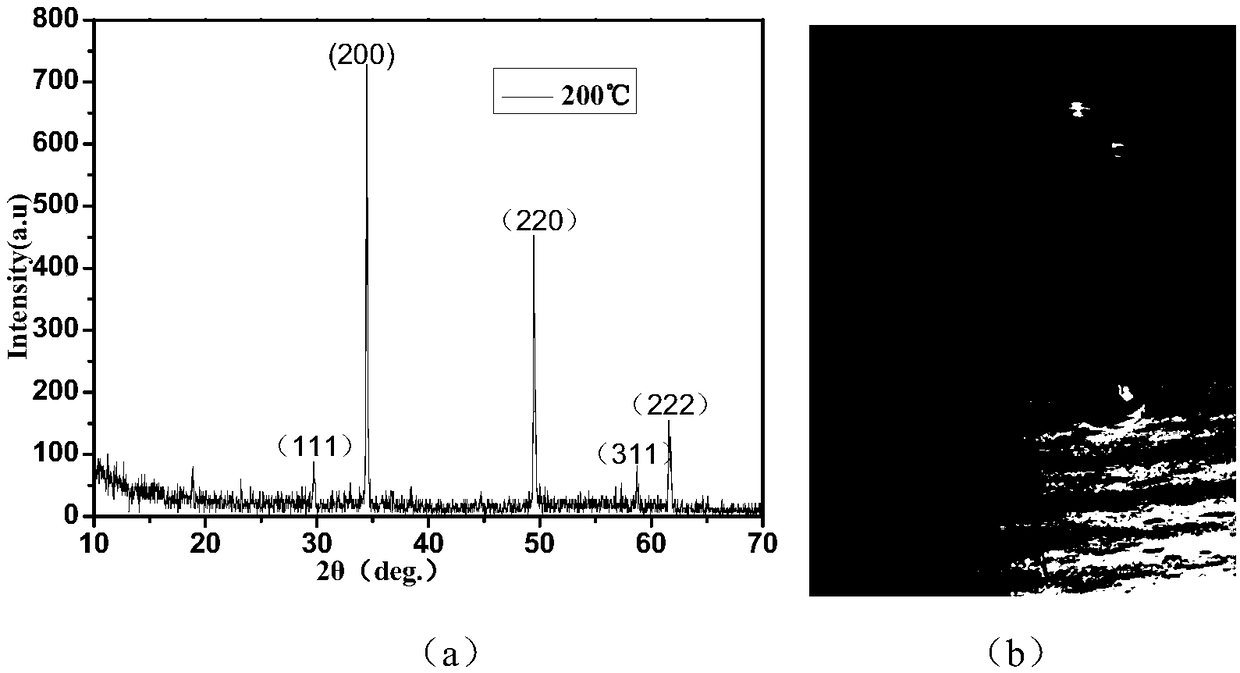
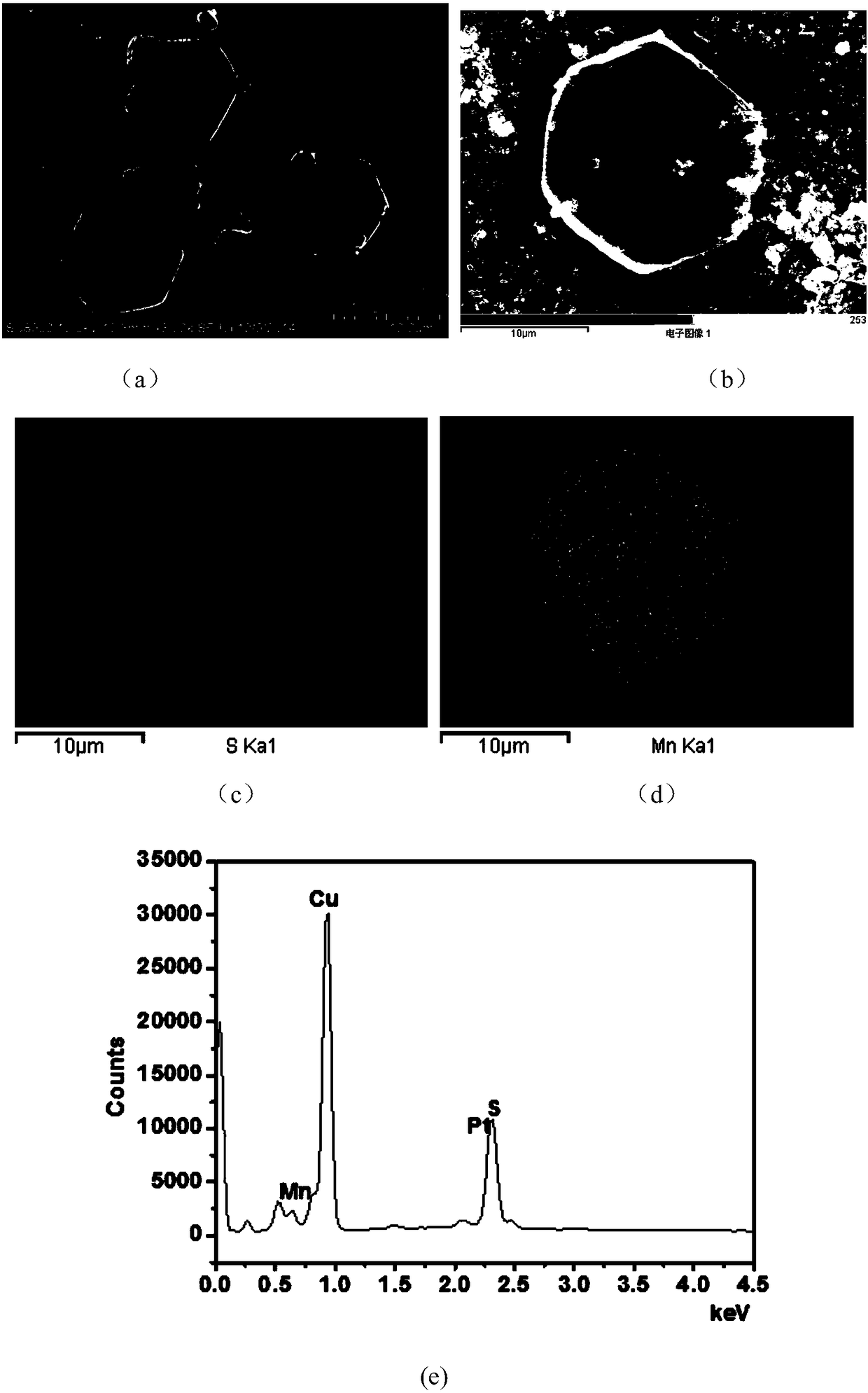
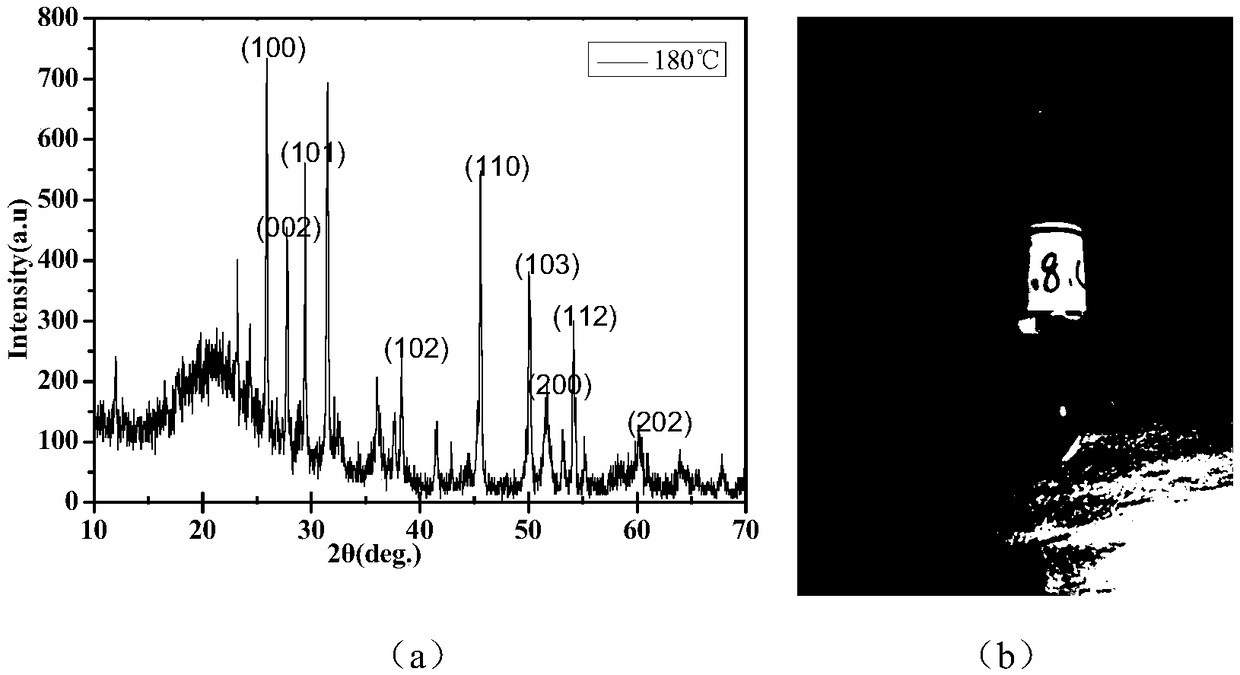
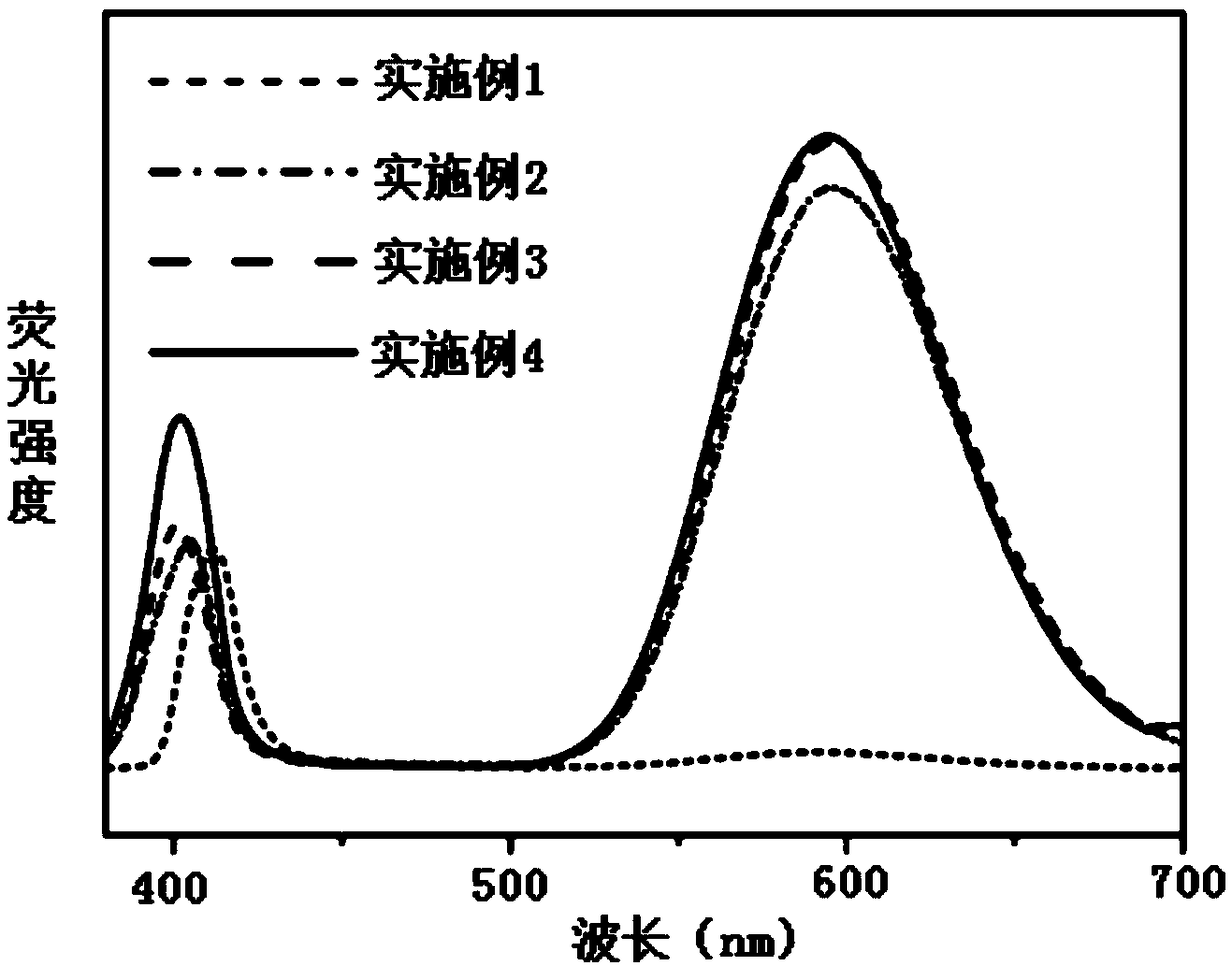
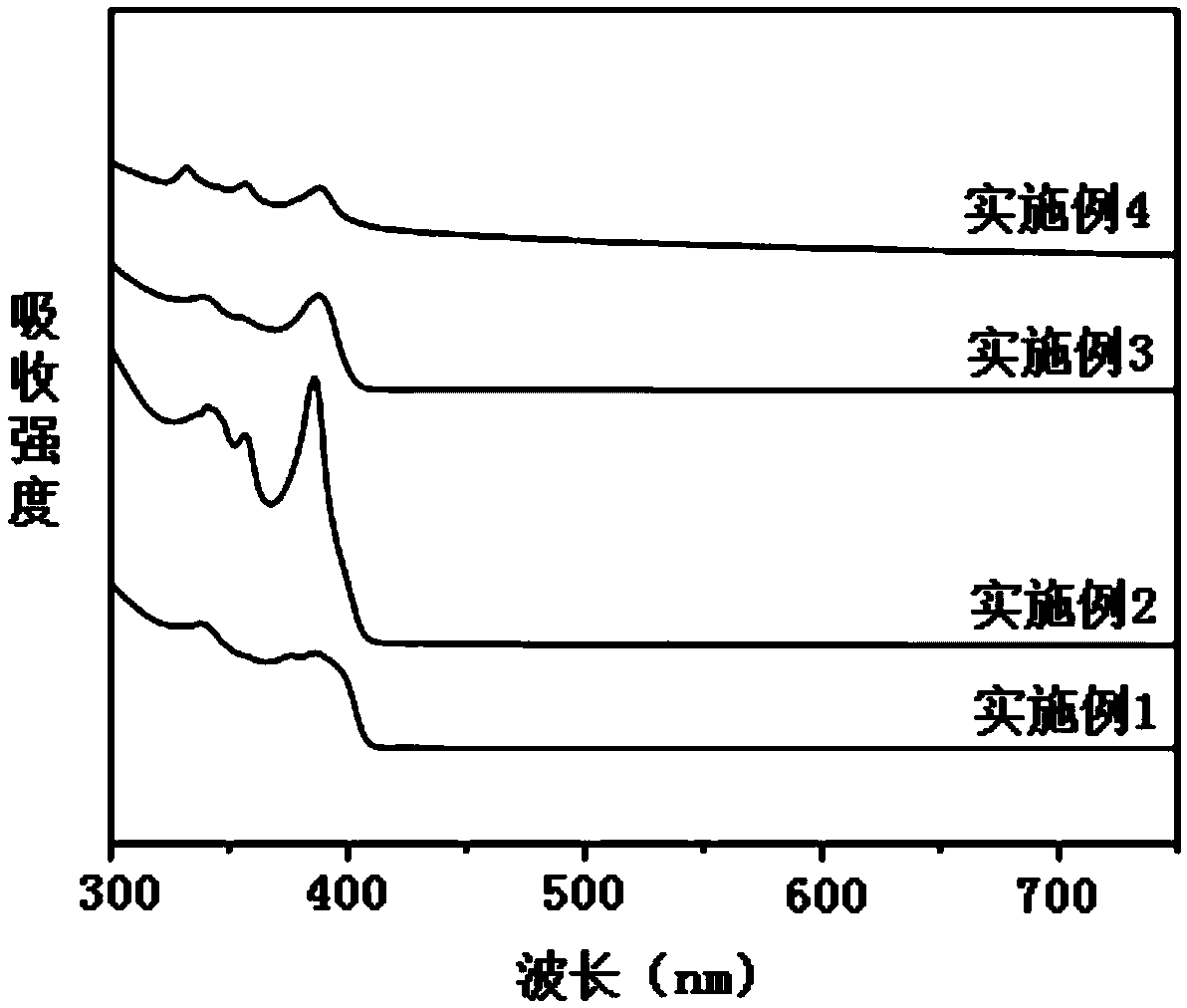
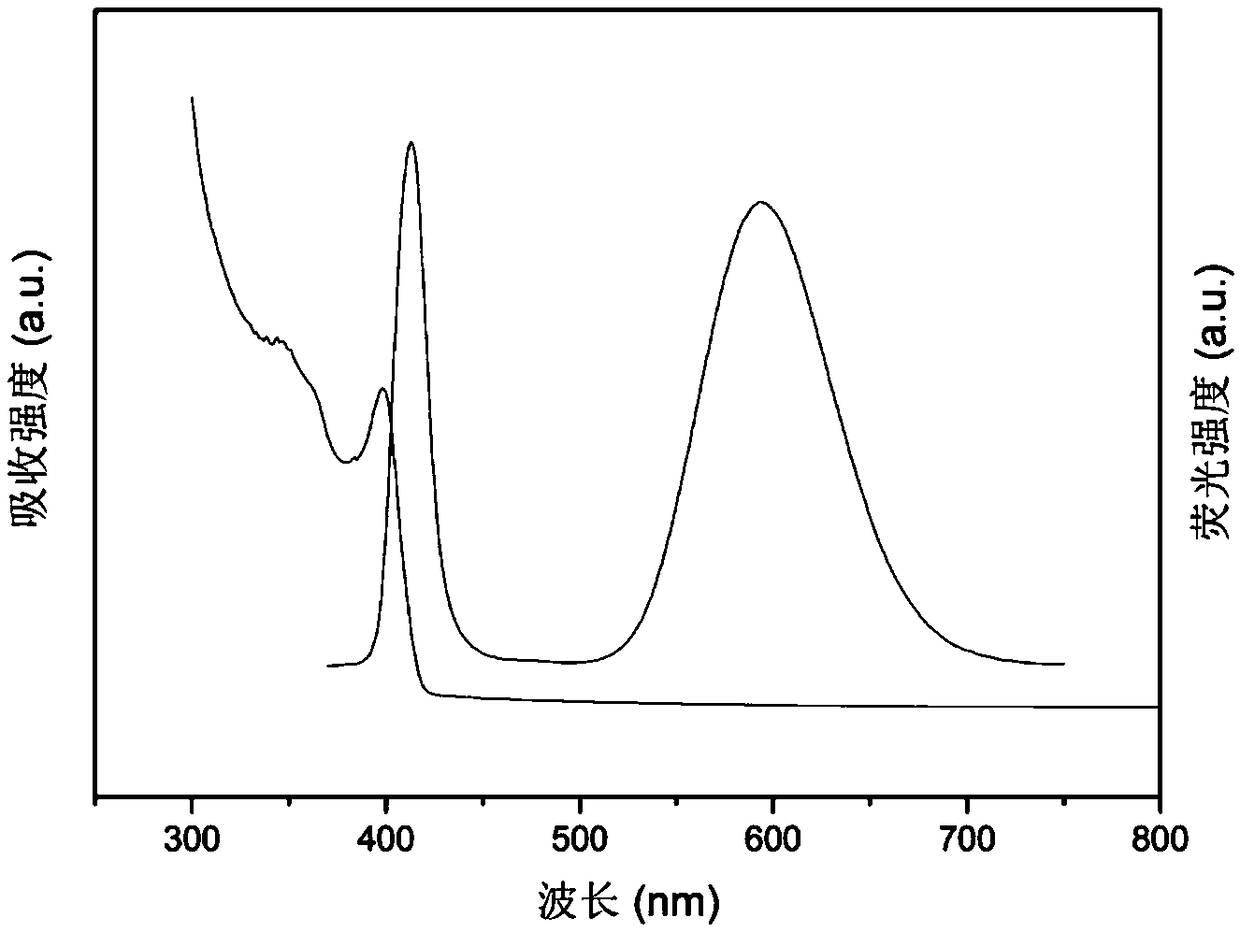
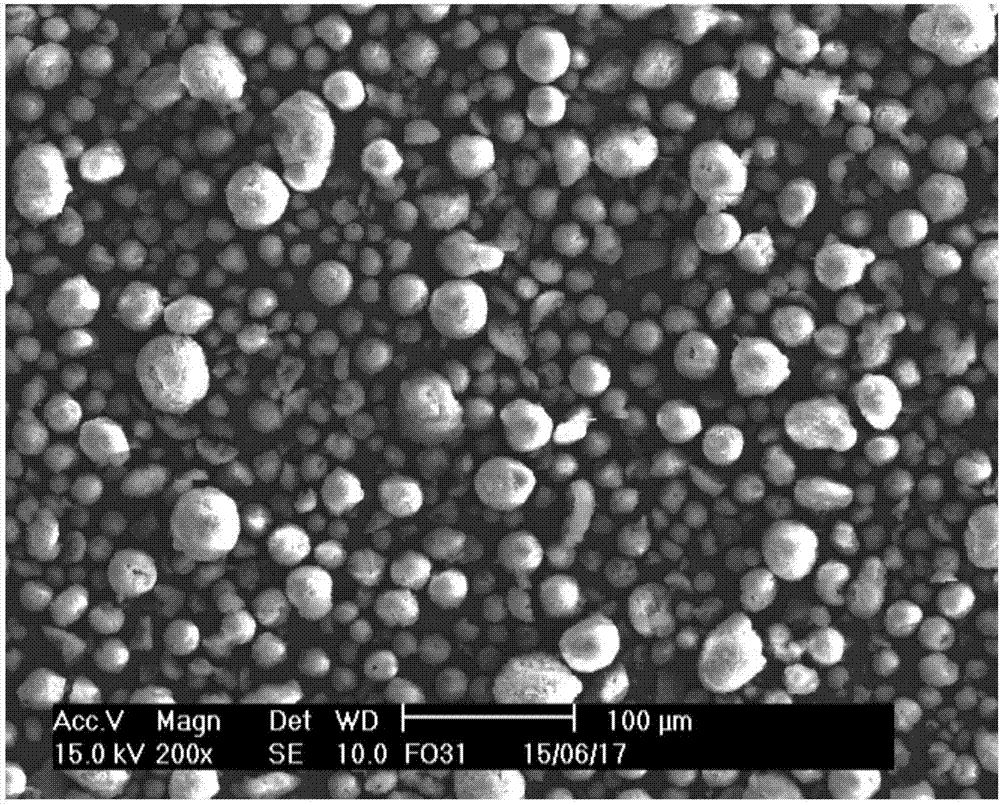
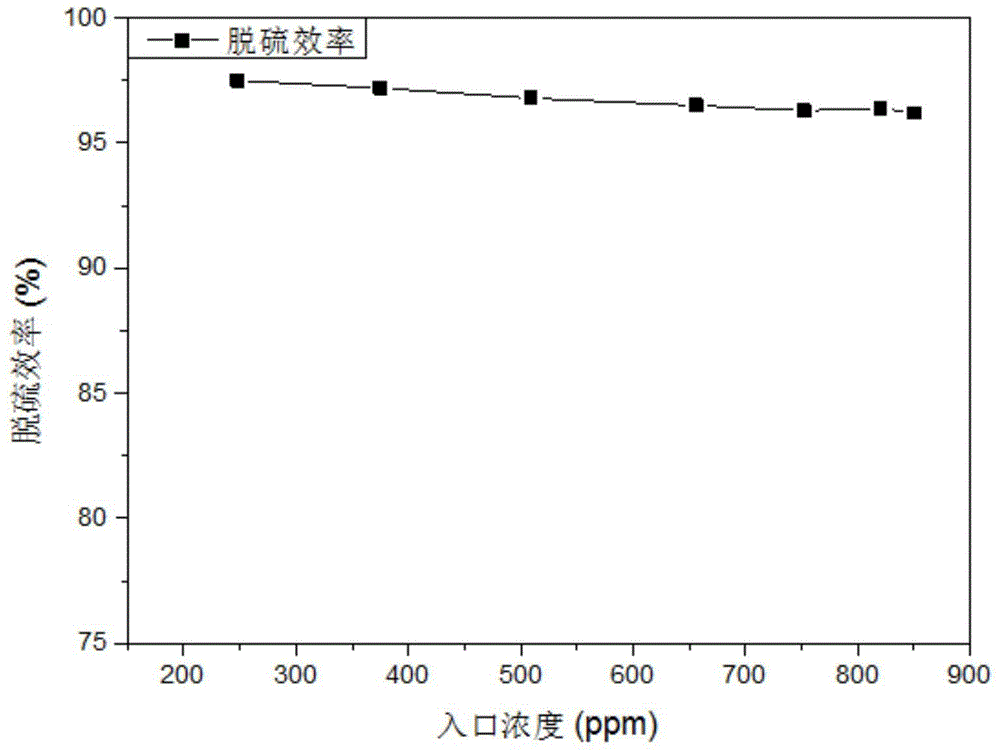
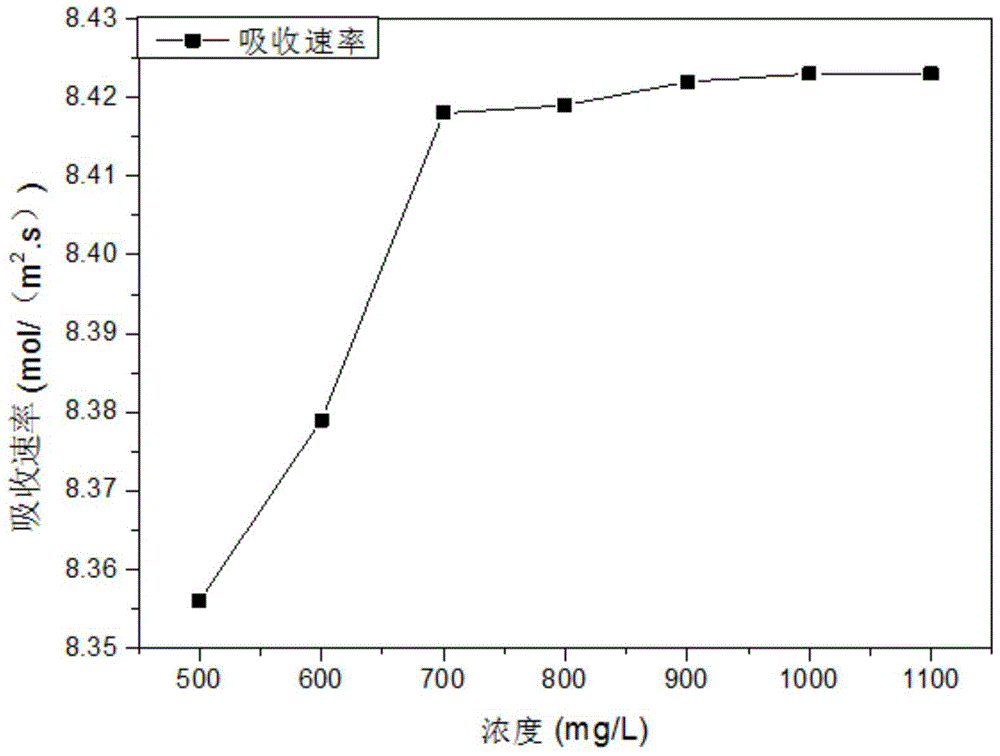
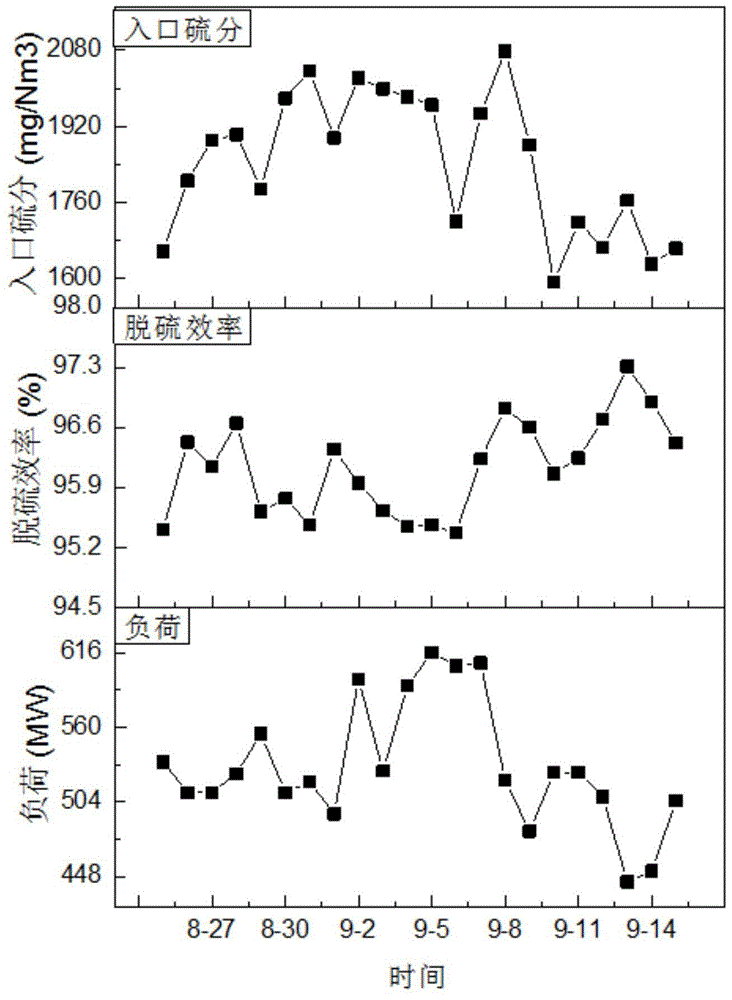
Patents
Literature
100 results about "Manganese(II) chloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Manganese(II) chloride describes a series of compounds with the formula MnCl₂(H₂O)ₓ, where the value of x can be 0, 2, or 4. The tetrahydrate is the most common form of "manganese(II) chloride" and is the tetrahydrate with the formula MnCl₂·4H₂O. The anhydrous form and a dihydrate MnCl₂·2H₂O are also known. Like many Mn(II) species, these salts are pink, with the paleness of the color being characteristic of transition metal complexes with high spin d⁵ configurations.
Synthesis method and application method of manganese Prussian blue analog material for lithium ion battery
InactiveCN109742398AControllable particle sizeControllable HollownessElectrode manufacturing processesFinal product manufactureSynthesis methodsPotassium ferricyanide
The invention discloses a synthesis method and application method of manganese Prussian blue material for a lithium ion battery, and belongs to the preparation and application method of the manganesePrussian blue material. The synthesis method comprises the following steps: selecting a manganese source from anhydrous manganese chloride (MnCl2) or manganese chloride monohydrate (MnCl2.H2O); selecting an iron source and cyanogen from potassium ferricyanide (K3[Fe(CN)6]); selecting a chelating agent from anhydrous sodium citrate or sodium citrate dihydrate; weighing the manganese source and theclelating agent in mass ratio of x: 1, and dissolving into a mixed solution with the methyl alcohol and deionized water in any proportion to prepare a solution A; dissolving the potassium ferricyanideinto the deionized water to prepare the solution B with the concentration of 0.04mol / L; pouring the solution B into the solution A, uniformly mixing to acquire the solution C, standing for 6-24h at the room temperature, and separating, purifying and drying to obtain a target product. The synthesis method disclosed by the invention has the advantages that the raw material is easy to obtain, the synthesis method is simple, the operation step is high in controllability, the obtained product is high in purity, and uniform in particle size; and the structure is a hollow cube and easy to prepare ina large-scale manner. The manganese Prussian blue material disclosed by the invention is served as the lithium ion battery negative material, and is excellent in electrochemical performance.
Owner:CHINA UNIV OF MINING & TECH
Fertilizer and pesticide combination reagent for preventing and killing bacterial canker of kivifruit and preparation method of fertilizer and pesticide combination
ActiveCN104628481AHigh affinityHigh activityNitrogenous fertilisersFertilizer mixturesLiquid productFermentation
The invention discloses a fertilizer and pesticide combination reagent for preventing and killing bacterial canker of kiwifruit and a preparation method of the fertilizer and pesticide combination reagent. The fertilizer and pesticide combination reagent is prepared from the following raw materials in parts by weight: 200 parts of agricultural amino acid water soluble powder, 40-50 parts of anhydrous zinc chloride, 45-65 parts of anhydrous manganese chloride, 40-80 parts of a ciprofloxacin active compound or a ciprofloxacin active compound, 100-200 parts of a 14% methyl chloride isothiazolinone water agent, 100-150 parts of oxine-copper active compound, 0.1 part of forchlorfenuron, 0.1 part of a 5-nitroguaiacol sodium salt active compound, 0.5 part of an alpha-pimacol active compound, 350-450 parts of molasses microbial fermentation concentrate solution, 0.2-0.25 part of sodium polyacrylate powder, 50-60 parts of No.YD-7 liquid fertilizer synergia solution, 0.1-0.2 part of industrial alcohol and the balance of deionized water to supplement the raw materials to 1200-1250 parts. The fertilizer and pesticide combination reagent disclosed by the invention is a high-density liquid product with fertilizer and pesticide effects, has good affinity for both water and plants, mainly contact and kill plant bacteria, and can also kill pathogenic fungus. A layer of compact organic pesticide and fertilizer film is formed outside a tree body when the fertilizer and pesticide combination reagent is applied in large concentration, and a sustained release function is exerted.
Owner:贵州三福生物科技有限公司 +1
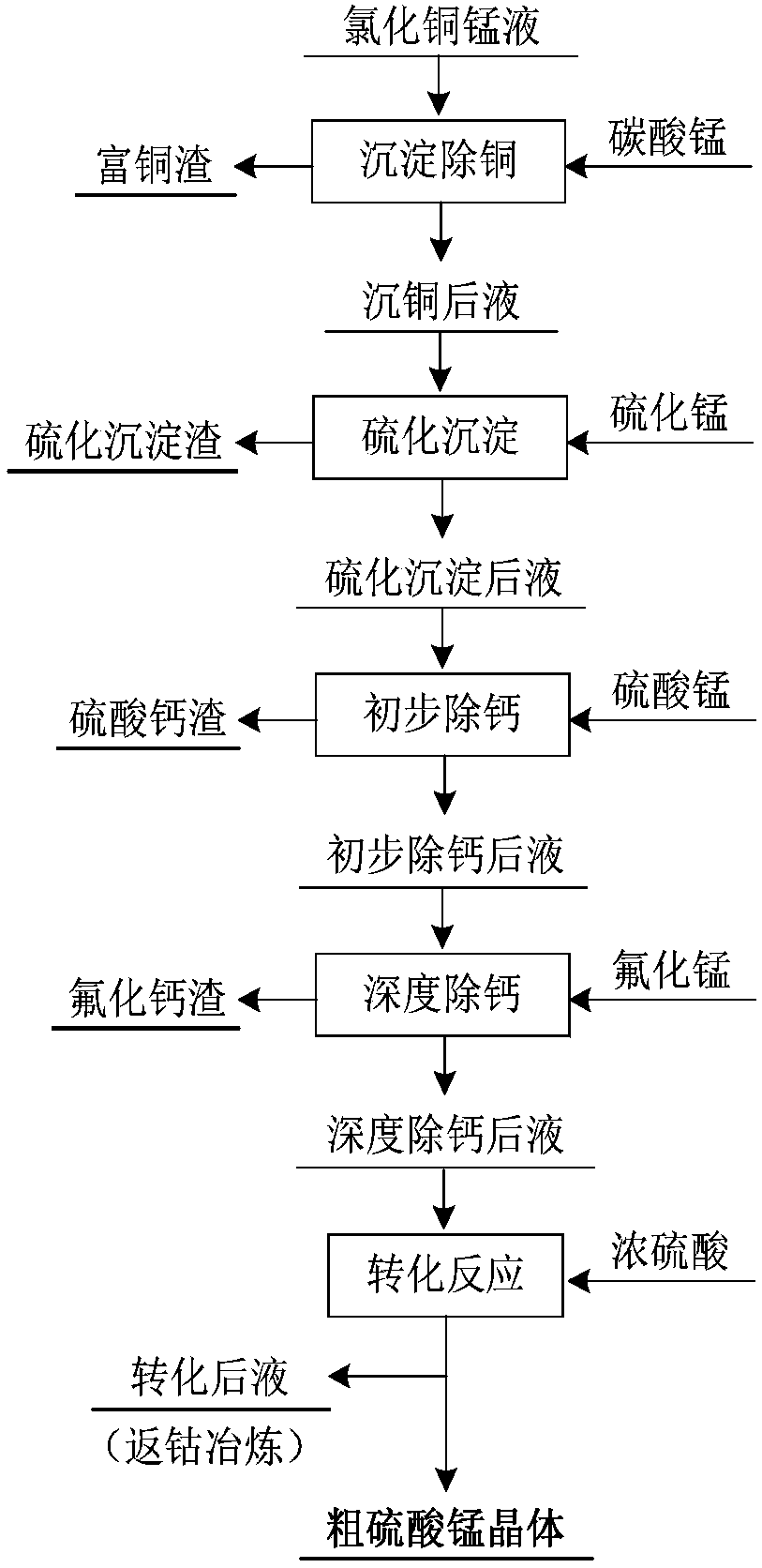
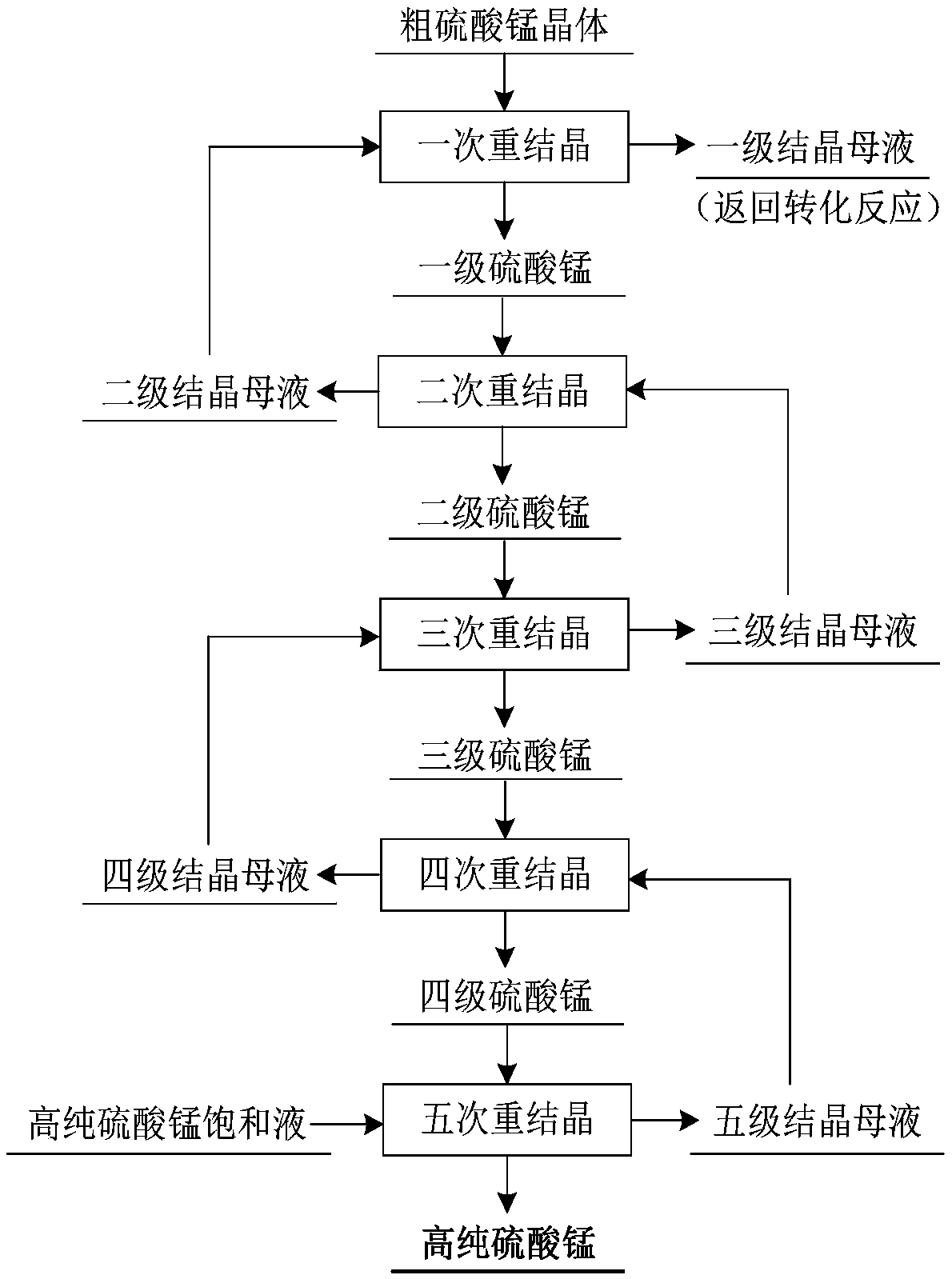
Method for preparing battery-grade manganese sulfate by using copper chloride manganese liquid
InactiveCN108585051ARealize deep decalcificationLow costManganese sulfatesWater dischargeDissolution
The invention discloses a method for preparing battery-grade manganese sulfate by using a copper chloride manganese liquid. The method comprises the steps that the copper chloride manganese liquid reacts with concentrated sulfuric acid to synthesize a coarse manganese sulfate crystal after neutralizing and precipitating copper ions through manganese carbonate, precipitating heavy metal ions through manganese sulfide, preliminarily precipitating calcium ions through manganese sulfate and deeply precipitating calcium ions through active manganese fluoride in sequence; and the coarse manganese sulfate crystal is recrystallized to obtain a battery-grade manganese sulfate crystal. The method for preparing the battery-grade manganese sulfate by using the copper chloride manganese liquid has theadvantages that the whole process adopts manganese-containing materials to remove and recover cuprum, zinc, calcium and other components in the copper chloride manganese liquid, and a five-stage countercurrent series recrystallization purification technology is combined, so that the quality of manganese sulfate is greatly improved, and the high-efficiency utilization of manganese resources and thewhole-process no-waste-water discharge are ensured; concentrated sulfuric acid is directly adopted to achieve the precipitation and conversion of manganese chloride to synthesize the coarse manganesesulfate, and the complex process for synthesizing the manganese sulfate by firstly extracting manganese ions then reversely extracting sulfuric acid or firstly precipitating manganese ions through carbonate then synthesizing the manganese sulfate through sulfuric acid dissolution is avoided.
Owner:CENT SOUTH UNIV
Trivalent chromium electroplate liquid capable of forming black clad layer
The invention discloses trivalent chromium electroplate liquid capable of forming a black clad layer. Each liter of the trivalent chromium electroplate liquid comprises the following raw materials: a cylinder opening agent, 20 to 150g of stabilizing agent, 10 to 20g of cobalt chloride or manganese chloride, 0.6 to 1.5g of ferric chloride, and 1 to 10g of wetting agent, wherein each liter of cylinder opening agent consists of 5 to 30g of blackening agent, 90 to 140g of chromic salt, 180 to 300g of L conducting salt, 25 to 90g of pH buffering agent. The trivalent chromium electroplate liquid capable of forming the black clad layer has simple components and is easy to maintain; and the formed black clad layer has deep black color and high adhesion.
Owner:GOOD CHEM SCI & TECH
Method for removing pentavalent antimony pollutants in water through manganese ion enhanced electrochemistry
ActiveCN104724797AImprove electrochemical performanceGood removal effectWater/sewage treatment by electrochemical methodsWater/sewage treatment using germicide/oligodynamic-processElectrochemical responseElectrochemistry
The invention belongs to the technical field of water treatment and in particular relates to a method for removing pentavalent antimony pollutants in water through manganese ion enhanced electrochemistry. The method comprises the following steps: installing an iron plate as an anode and a steel plate as a cathode in an electrochemical reactor; adjusting the pH value of to-be-treated water containing the pentavalent antimony pollutants to 4-8, introducing the to-be-treated water into the electrochemical reactor, adding a manganese sulfate solution or a manganese chloride solution at a concentration of 0.1-5.0mmol / L and adjusting the current density to 0.5-10mA / cm<2>; carrying out electrochemical treatment for 5-20 minutes and supplying power by adopting a direct current electrical source. The pentavalent antimony pollutants can be rapidly and effectively removed by adopting the electrochemical method.
Owner:RES CENT FOR ECO ENVIRONMENTAL SCI THE CHINESE ACAD OF SCI
Method for removing magnesium impurity element in waste aluminum regeneration
ActiveCN103740948AWon't affect yieldThe impurity element magnesium is reducedProcess efficiency improvementAluminum fluorideNonferrous metal
The invention relates to a method for removing a magnesium impurity element in waste aluminum regeneration and belongs to the technical field of nonferrous metal regeneration. The method comprises the following steps: mixing 10 to 30 percent of manganese chloride, 10 to 30 parts of calcium chloride, 10 to 30 parts of aluminum fluoride, 5 to 20 percent of active carbon, less than or equal to 20 percent of calcium oxide and 5 to 20 percent of magnesium carbonate into a stirring vessel to obtain magnesium removal solvent; smelting waste aluminum in a smelting furnace to waste aluminum melt, adding the magnesium removal solvent, standing the mixture after the magnesium removal solvent is added to obtain waste aluminum melt with the magnesium removal solvent; removing the magnesium oxide and / or magnesium compound in a slagging-off manner when the magnesium oxide and / or magnesium compound in the waste aluminum melt floats to the surface of the waste aluminum melt; then standing the mixture, pouring after the mixture is discharged from the furnace to obtain the regenerated aluminum alloy with the magnesium impurity element in the waste aluminum being removed. By adopting the method, the yield of the aluminum is not influenced, the impurity elemental magnesium of the aluminum melt is remarkably reduced; the environment is not damaged; the process flow is short, and the industrialized production requirement can be met.
Owner:HUAWEI TEHCHNOLOGIES CO LTD
N-doped carbon-supported base metal oxygen reduction/oxygen evolution dual-function catalyst
InactiveCN106252674AObvious cost advantageSimple and fast operationCell electrodesHeat treatedLamellar structure
The invention discloses an N-doped carbon-supported base metal oxygen reduction / oxygen evolution dual-function catalyst. The preparation method comprises the steps of taking proper amount of acetylene black to be subjected to strong oxidation treatment, and then attaching the proper amount of manganese chloride on the surface of the acetylene black and implementing heat treatment in the atmosphere of ammonia gases to prepare N-doped acetylene black; and adding the N-doped acetylene black into ethanol-aqueous solution of nickel nitrate, ferric nitrate and urea to implement hydrothermal reaction so as to uniformly and naturally embed nickel and iron hydroxide crystals of lamellar structures into the N-doped acetylene black, thus acquiring the N-doped carbon-supported base metal oxygen reduction / oxygen evolution dual-function catalyst, wherein the N-doped acetylene black plays a role in oxygen reduction and catalysis, and nickel and iron hydroxide crystals play a role in oxygen evolution and catalysis. The catalyst can solve the problem about preparation and compounding of the N-doped carbon material and metal hydroxide respectively used as oxygen reduction and oxygen evolution reaction catalyst, so that the catalyst3 simultaneously is good in catalytic activity and compounding performance, low in cost and prone to produce in large scale.
Owner:SICHUAN UNIVERSITY OF SCIENCE AND ENGINEERING
Preparation method and applications of carbon nano-tube-supported transition metal oxide material
InactiveCN108682868AHigh strengthImprove toughnessFuel and primary cellsCell electrodesFiltrationCarbon nanotube
The invention relates to a preparation method and applications of a carbon nano-tube-supported transition metal oxide material. The preparation method comprises: annealing CNTs, dissolving, carrying out ultrasonic treatment, and carrying out suction filtration and film forming; dissolving 1.74 g of cobalt nitrate hexahydrate, 0.436 g of nickel nitrate hexahydrate, manganese chloride, ammonium fluoride, urea and cetyltrimethylammonium bromide in 80 ml of deionized water, wherein a molar ratio of cobalt nitrate hexahydrate to manganese chloride is 0.05-0.4, and a molar ratio of cobalt nitrate hexahydrate to ammonium fluoride to urea to cetyltrimethylammonium bromide is 6:9:20:4; and transferring the formed solution and the carbon nano-tubes into a reaction kettle, and preparing the carbon nano-tube-supported transition metal oxide material through a hydrothermal method. According to the present invention, the preparation process is simple, the cost is low, the prepared material has advantages of good structure uniformity, large specific surface area and excellent electrochemical performance, and the disadvantages that the cost of the precious metal catalyst is high, the preparation process is complicated and the industrialization is difficult to achieve are overcome.
Owner:UNIV OF SHANGHAI FOR SCI & TECH
Anisotropic wurtzite MnSe nanocrystal synthesized by utilizing solvothermal method
InactiveCN102633240AHigh crystallinityUniform particle size distributionNanotechnologyBinary selenium/tellurium compoundsFiberSolvent
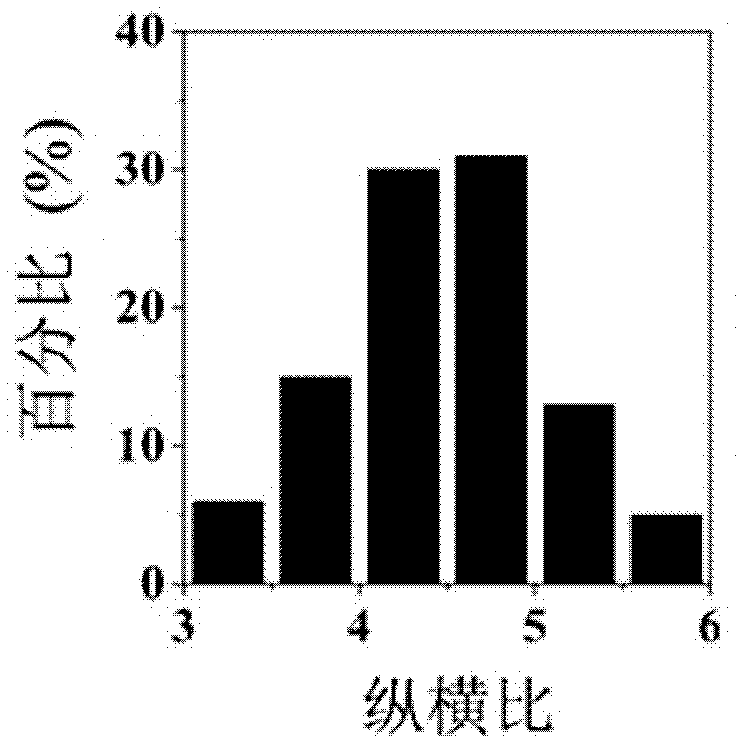
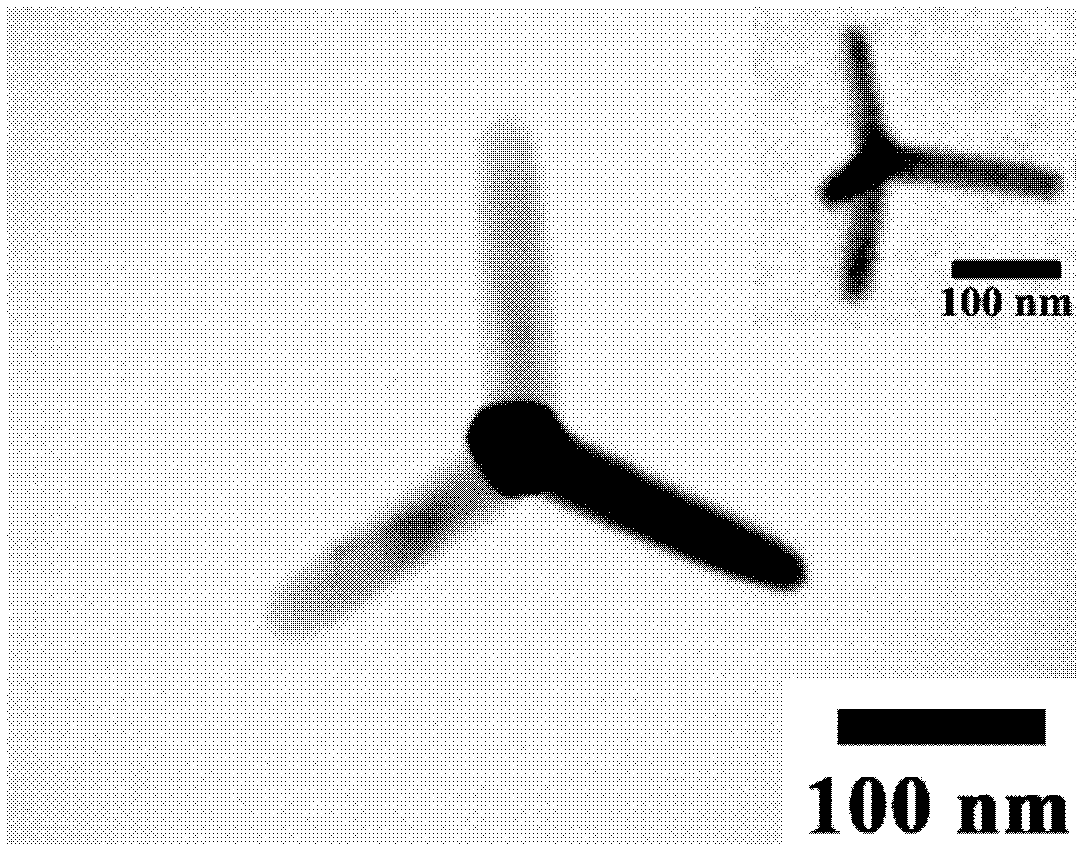
The invention discloses an anisotropic wurtzite MnSe nanocrystal synthesized by utilizing a solvothermal method, belonging to the technical field of nanometer material preparation. Synthetization is carried out under the protection of nitrogen in a schlenk system; manganese chloride and selenium powder are taken as raw materials, oleyl amine and oleic acid are used as ligands; the raw materials and the ligands are placed into a three-necked bottle to be stirred and heated until a clarified faint yellow solution is obtained; the solution is heated to the temperature of 290-320 DEG C in a heating rate of 2-25 DEG C / min; and the temperature is kept for 30-60 minutes to obtain an anisotropic four-footed shape wurtzite MnSe nanocrystal or / and an anisotropic water-drop wurtzite MnSe nanocrystal. The sample prepared by the invention is high in phase purity, good in crystallinity and uniform in particle size distribution. The preparation method is simple in process, short in synthesized time, controllable in morphology and aspect ratio of the product and high in repeatability and the like.
Owner:JILIN UNIV
Manufacturing method of ultra-pure battery-grade manganese chloride for battery material
The invention relates to a manufacturing method of ultra-pure battery-grade manganese chloride for a battery material, which comprises the following steps that: manganese chloride solution which is prepared by leaching ordinary manganese carbonate or metal manganese with high impurity content through hydrochloric acid is added with hydrofluoric acid, fluoride, phosphoric acid, phosphate salt or polyphosphoric compound to remove calcium (Ca) and magnesium (Mg), sulfide, polysulfide or sulfuric organic sodium salt is used for removing heavy metal impurities, the pH value of the solution is maintained at 2.5 to 6.0, the solution is filtered after the reaction, filtered liquid enters an iron-removing reactor, the reaction temperature is controlled at 30 to 120 DEG C, the Baume degree is 30 to56 degrees Be, industrial peroxide or hydrogen peroxide is added under the pH value of 2.5 to 6.0 to remove the iron, organic matters can be removed by adding particle or powder active carbon under the temperature of 30 to 120 DEG C, filter liquid after the reaction and filter is pumped into a concentrator to be concentrated, crystallized and dripped at a normal pressure or a negative pressure, so an ultra-pure battery-grade manganese chloride product is obtained. For the manganese chloride product prepared through the method, the content of the manganese chloride product can reach more than 99 percent.
Owner:HUBEI HAOYUAN MATERIAL TECH
Preparation method of cathode material lithium nickel cobalt manganese oxide of lithium battery
InactiveCN106410118AImprove securityImprove high temperature performanceCell electrodesAutomatic controlManganese oxide
The invention aims to provide a preparation method of a cathode material lithium nickel cobalt manganese oxide of a lithium battery. Soluble nickel, cobalt and manganese salt are taken as raw materials, and the method is characterized by comprising specific process steps as follows: 1) preparation of a nickel-cobalt-manganese precursor through coprecipitation: soluble nickel, cobalt sulfate and manganese chloride are taken as the raw materials, water is added, a nickel, cobalt and manganese salt mixed aqueous solution is prepared, a sodium hydroxide aqueous solution is added as a precipitant, ammonia water is taken as a complexing agent, coprecipitation is performed under conditions of heating and alkali presence, a filtrate and filer residues are obtained through filtering, the filter residues are cleaned, and the nickel-cobalt-manganese precursor is prepared; 2) preparation of a lithium nickel cobalt manganese oxide product through mixing: the prepared nickel-cobalt-manganese precursor is dried, ground and stirred and mixed sufficiently with lithium salt, the mixture is loaded in a bowl, calcined, sintered, broken screened, dried and packaged, and the cathode material lithium nickel cobalt manganese oxide is obtained; the calcining and sintering time and the sintering temperature are controlled. Full-automatic controlled production is adopted in the production process, the production operation is convenient, reaction conditions are mild, and the prepared ternary cathode material lithium nickel cobalt manganese oxide has excellent performance.
Owner:JIANGXI JIANGTE LITHIUM LON BATTERY MATERIAL
Manganese vanadate nanoneedle structure and synthesis method thereof
InactiveCN103147128AAchieve synthesisLow costPolycrystalline material growthFrom normal temperature solutionsSynthesis methodsSolvent
The invention discloses a manganese vanadate nanoneedle structure and a synthesis method thereof, belonging to the technical field of preparation of nano materials. The manganese vanadate nanoneedle structure disclosed by the invention is composed of an MnV2O5 orthorhombic phase which is about 10 mu m long, and the diameter of the nanoneedle tip is about 50 nm. The synthesis method comprises the following steps: by using manganese chloride and sodium vanadate as raw materials, sodium dodecylsulfate (SDS) as a surfactant and water as a solvent, evenly mixing the manganese chloride, sodium vanadate, SDS and water, sealing the mixture in a reaction vessel, and keeping at the temperature of 120-180 DEG C for 6-24 hours, thereby finally obtaining the brown flocculent manganese vanadate nanoneedle structure, wherein the amount of the manganese chloride and sodium vanadate is not greater than 10 wt% of the water, and the amount of the SDS is not greater than 10 wt% of the water. The invention has the advantages of simple synthetic process, and no environmental pollution by the raw materials and synthetic process, conforms to the trend of modern industry requiring environmental protection, and can implement mass synthesis of the manganese vanadate nanoneedle structure.
Owner:ANHUI UNIVERSITY OF TECHNOLOGY
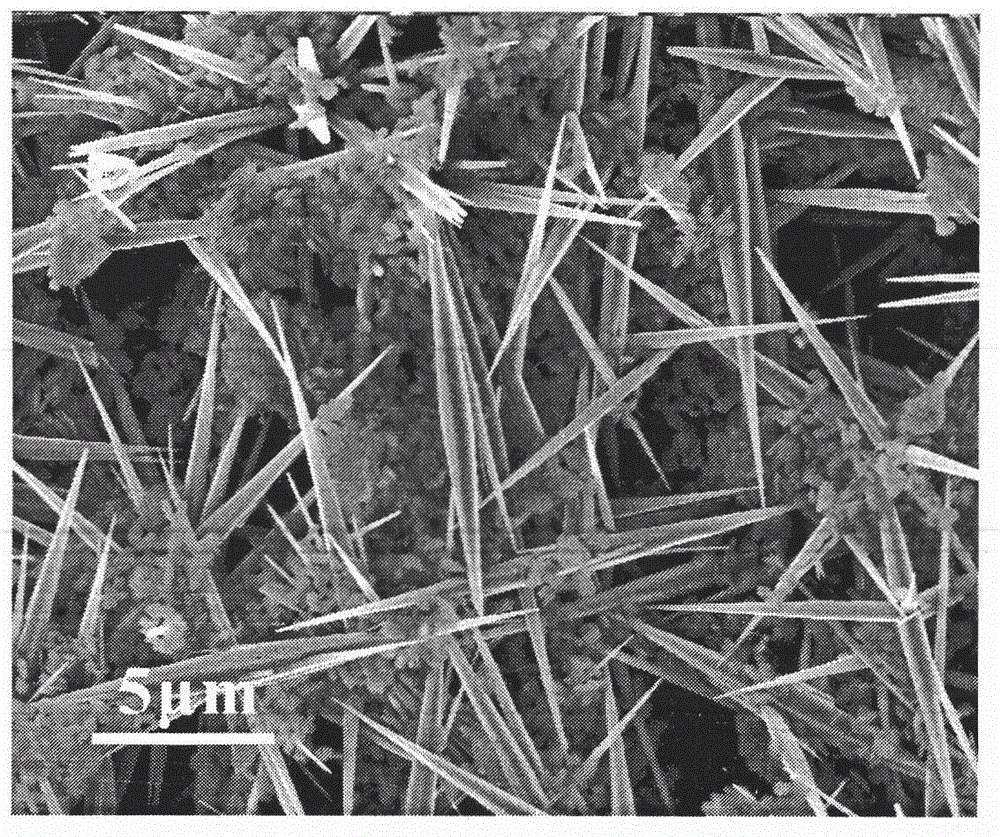
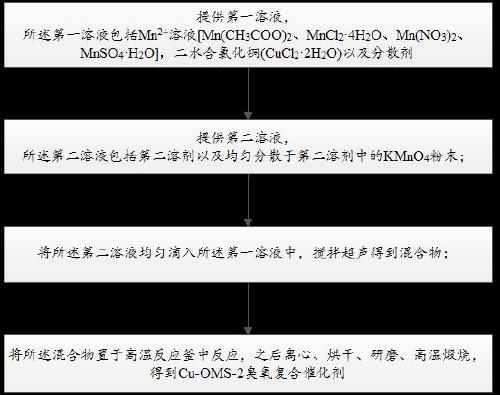
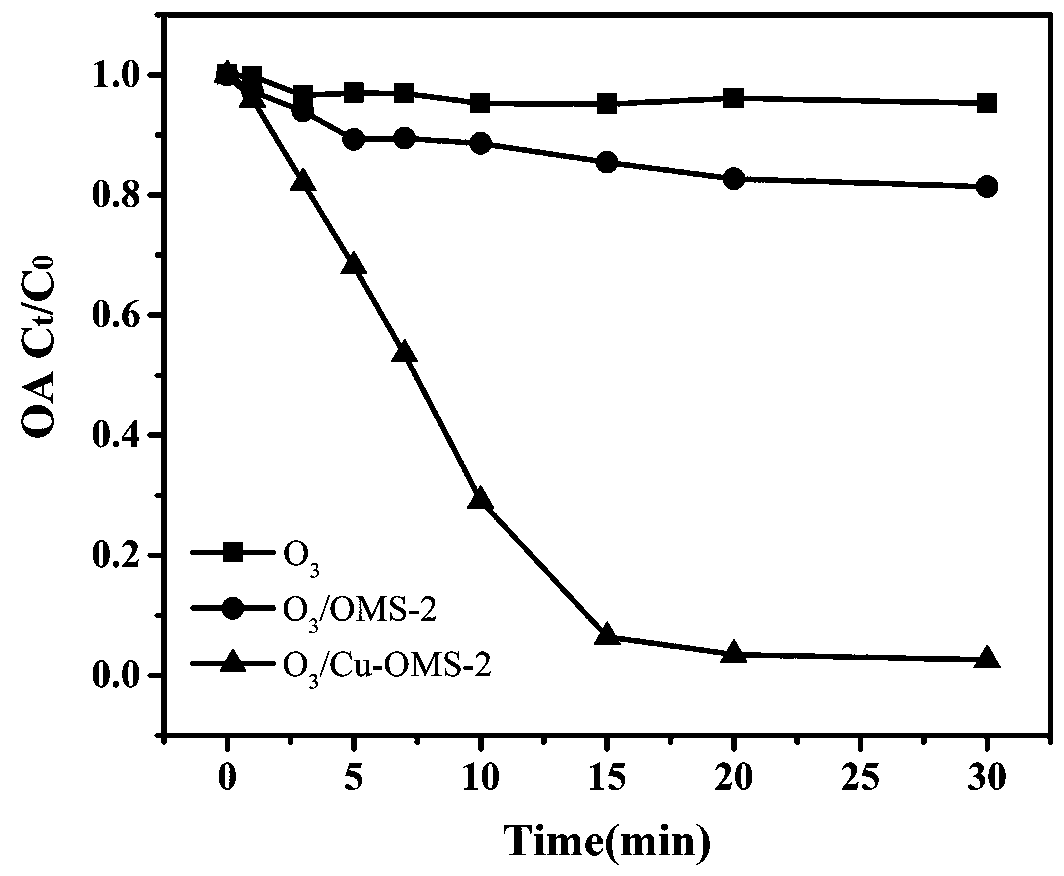
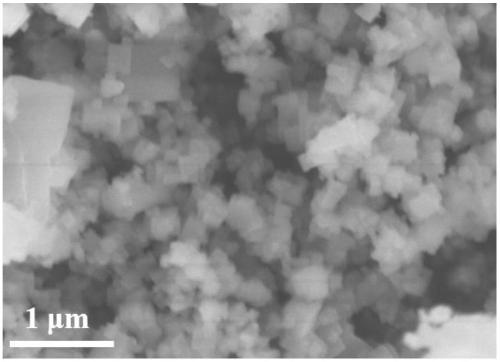
Preparation method and application of rod-like ozone catalyst Cu-OMS-2
InactiveCN110314689APromote degradationGood removal effectWater contaminantsCatalyst activation/preparationMANGANESE ACETATEAcetic acid
The invention provides a preparation method and application of a rod-like ozone catalyst Cu-OMS-2. The preparation method includes: providing a first solution, wherein the first solution comprises a Mn<2+> solution [such as manganese acetate (Mn(CH3COO)2), manganese chloride (MnCl2 4H2O), manganese nitrate (Mn(NO3)2) and manganese sulfate MnSO4 H2O], copper chloride dihydrate (CuCl2 2H2O) and a dispersing agent; providing a second solution, wherein the second solution comprises a second solvent and potassium permanganate (KMnO4) powder evenly dispersed in the second solvent; dropwise adding the second solution into the first solution, and stirring and performing ultrasonic treatment to obtain a mixture; placing the mixture into a high-temperature reaction kettle to perform reaction, centrifuging, drying, grinding, and calcining at high temperature to obtain the Cu-OMS02 ozone compound catalyst. Cu<2+> is doped into an OMS-2 crystal structure and then used for ozone catalysis, metal iondissolution after traditional metal catalyst reaction is lowered effectively, and catalyst activity and stability are increased.
Owner:WUHAN TEXTILE UNIV
Preparation method and application of sodium-rich anhydrous prussian blue analogue material
InactiveCN109292795ALow costImprove responseIron cyanidesCell electrodesFiltrationSodium-ion battery
The invention relates to a sodium-rich anhydrous prussian blue analogue material. The preparation method comprises the following steps: using potassium ferrocyanide or sodium ferrocyanide, sodium chloride and manganese chloride as precursors for preparing a prussian blue analogue, so that a turbid liquid is formed; collecting solid materials in the turbid liquid by using a centrifugation and suction filtration method, and carrying out water washing until no residual sodium chloride exists in the product, so that a sodium-rich prussian blue analogue is obtained; grinding the prepared sodium-rich prussian blue analogue into powder, laying the powder in a square boat, and placing the square boat in a constant-temperature zone of a tubular furnace for calcining; and taking one or a mixture ofmore selected from N2, He an Ar as an inert gas source, heating the tubular furnace to 180 DEG C at a heating speed of 1-10 DEG C / min, carrying out heat preservation for 2-3 hours to remove interstitial water, and carrying out cooling to room temperature along with the furnace after the heat preservation is finished, so that the sodium-rich anhydrous prussian blue analogue material is obtained. The prepared composite material of prussian blue analogue is applied to positive poles of sodium-ion batteries.
Owner:TIANJIN UNIV
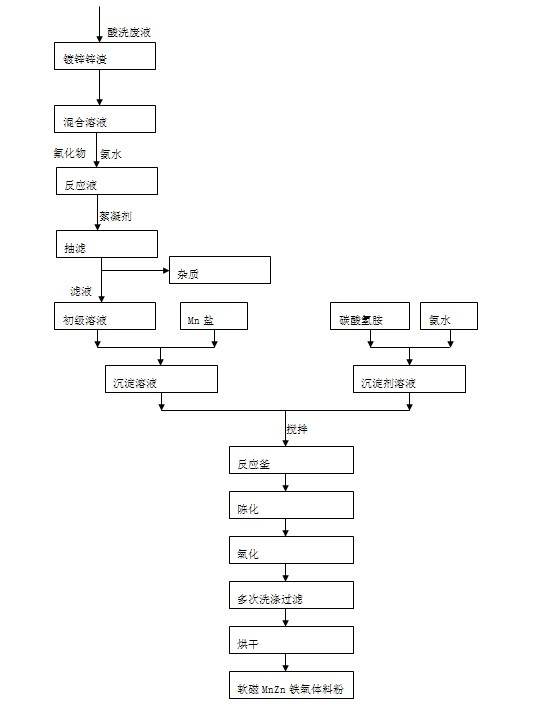
Method for preparing MnZn ferrite material powder from acid washing waste liquid and galvanized zinc slag
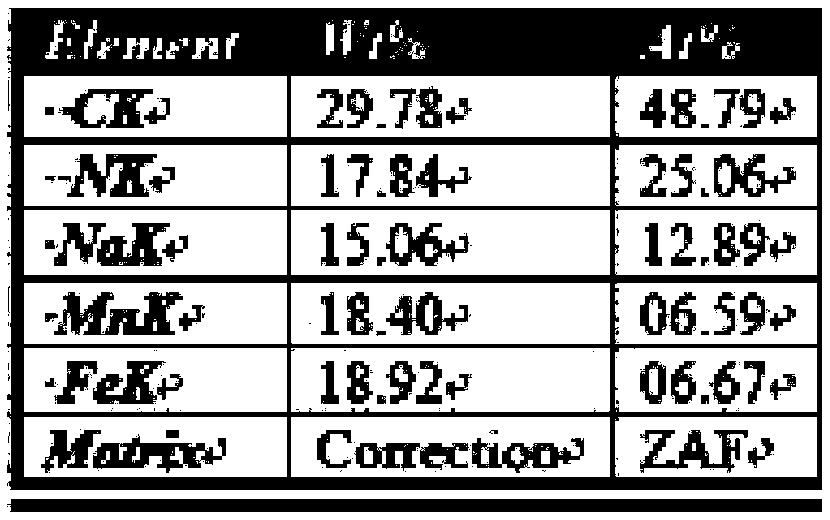
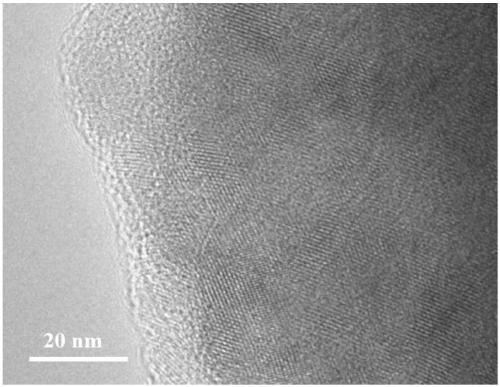
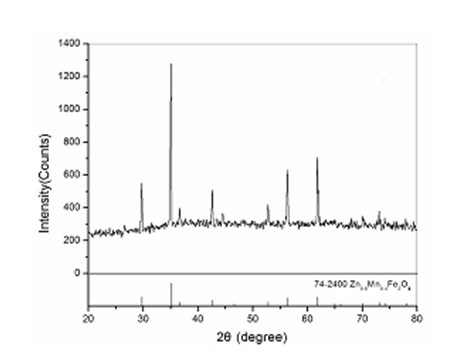
The invention discloses a method for preparing MnZn ferrite material powder from acid washing waste liquid and galvanized zinc slag. The method comprises the following steps of: (1) placing the galvanized zinc slag into the acid washing waste liquid to dissolve to obtain mixed solution; (2) adjusting the pH value of the mixed solution to 3 to 6 by using ammonia water, adding fluoride and reacting to obtain reaction solution; (3) adding a flocculating agent into the reaction solution and filtering out precipitate in the mixed solution by a suction filtration method, wherein the filtrate is primary solution; (4) preparing precipitate solution from the primary solution and manganese chloride according to the mole ratio of Fe2O3 to MnO to ZnO of the ferrite components and preparing precipitator solution from ammonium bicarbonate and ammonia water; (5) allowing the precipitate solution and the precipitator solution to enter a reaction kettle to react to obtain slurry; (6) ageing the slurry obtained from the complete reaction; and (7) oxidizing, washing, filtering and drying the aged slurry to obtain MnZn ferrite material powder. The soft magnetic MnZn ferrite material powder has fine granularity, uniform and stable components and high magnetic property.
Owner:HBIS COMPANY LIMITED HANDAN BRANCH COMPANY
High-sensitivity bimodal magnetic resonance contrast agent and preparation method thereof
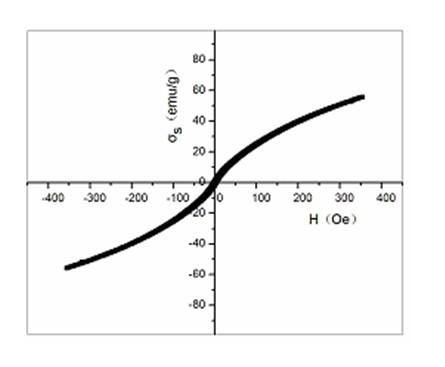
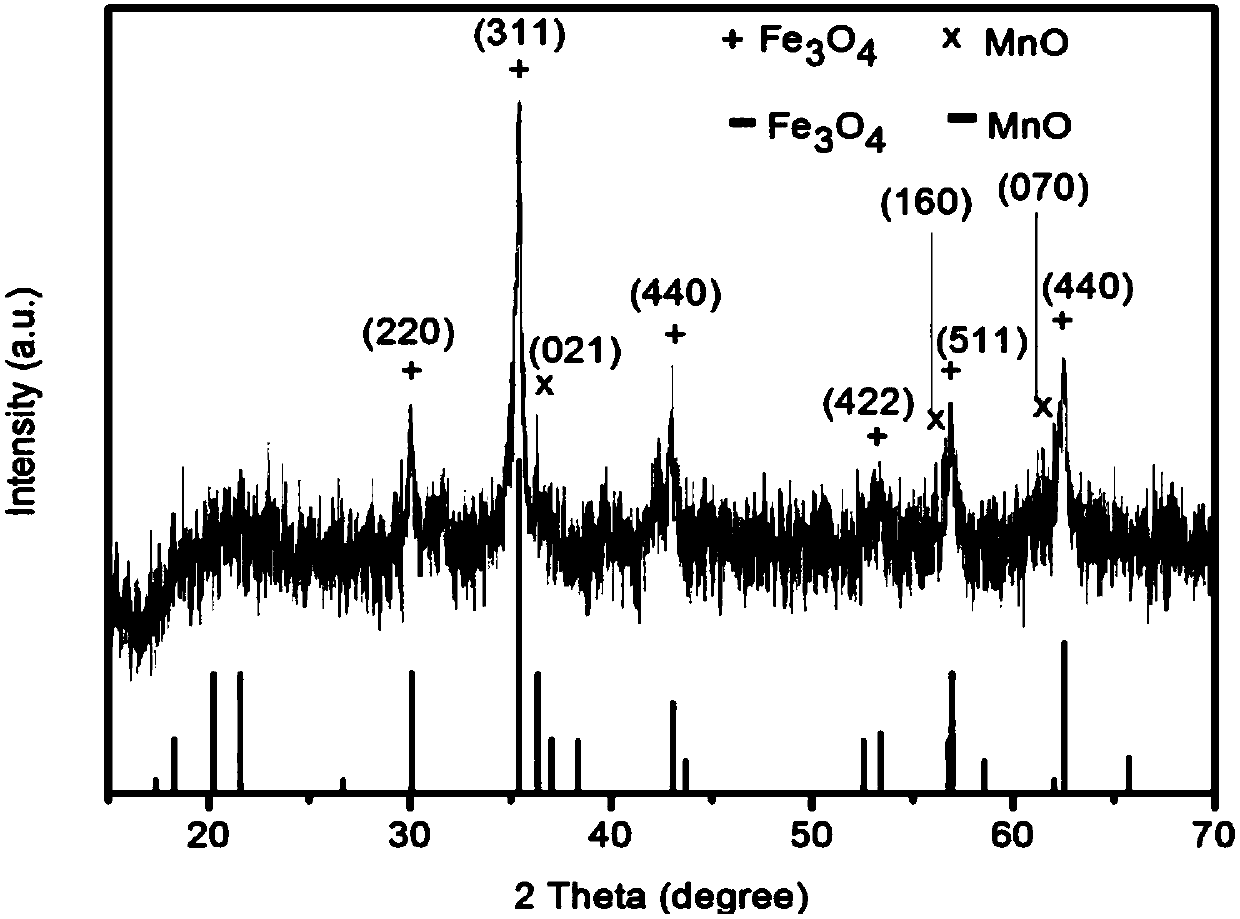
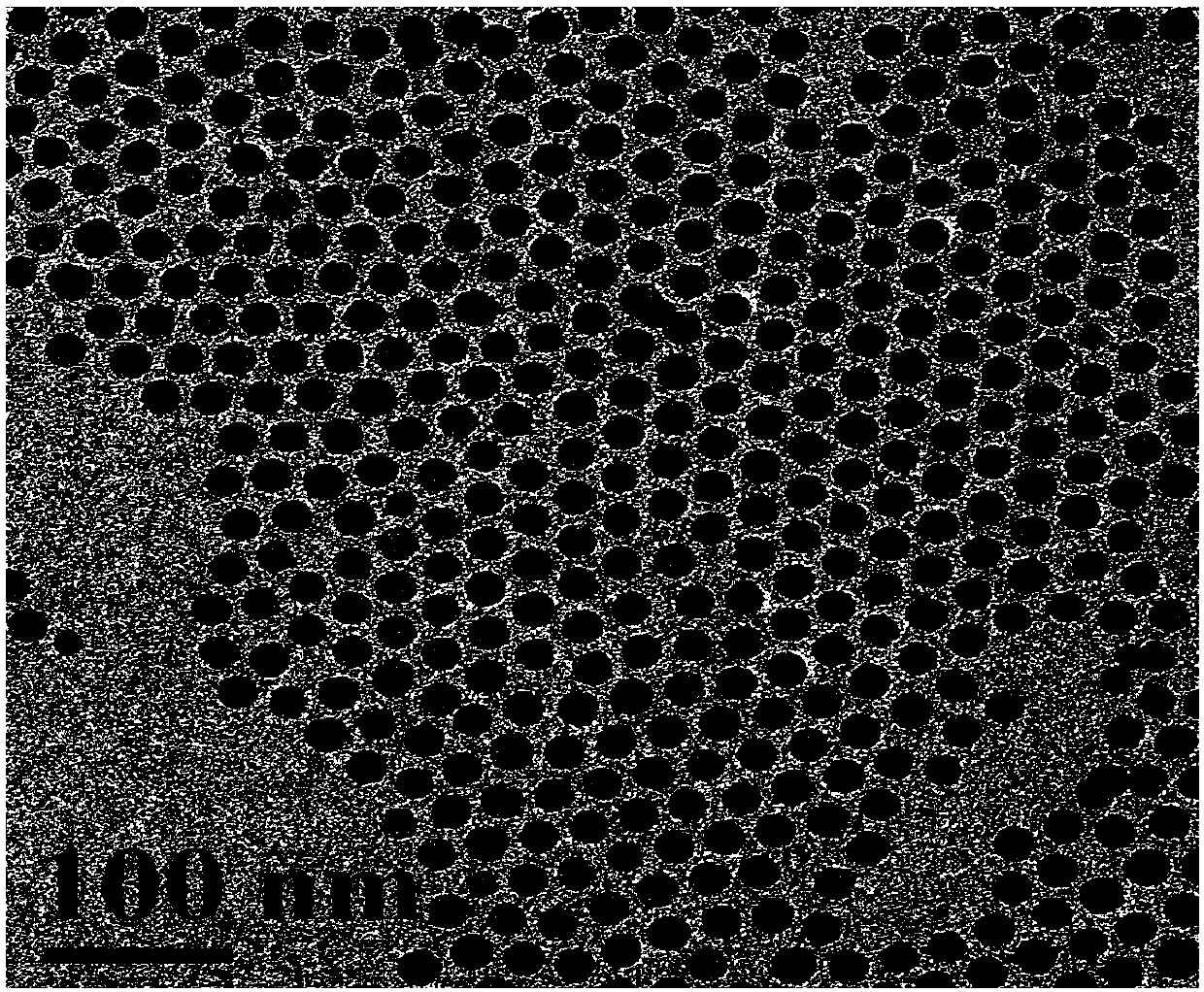
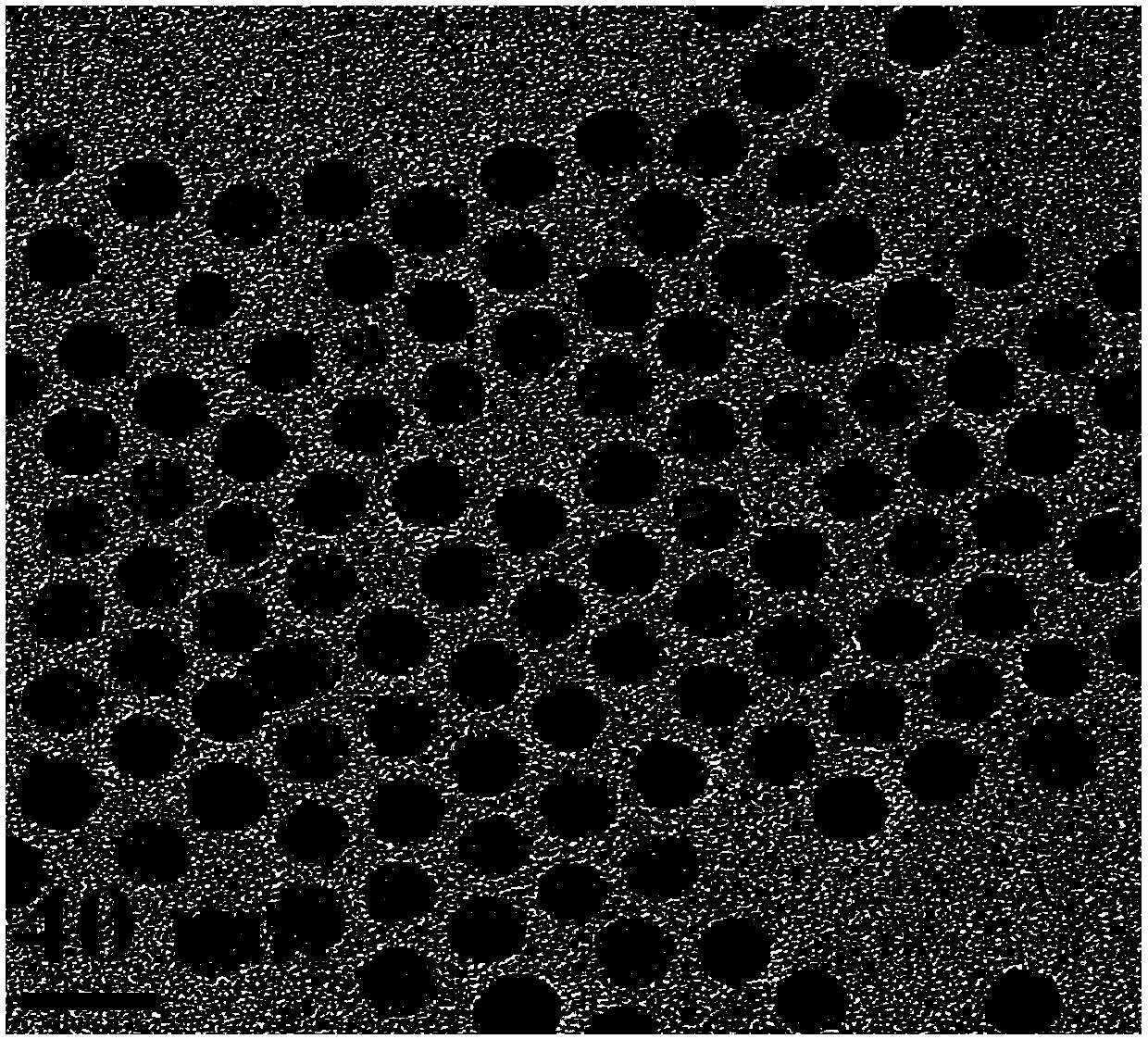
ActiveCN108030933AAccurate diagnosisEnhancing the Effects of Dual-Modal Magnetic Resonance ImagingGeneral/multifunctional contrast agentsNanomedicineChemical synthesisSuperparamagnetic iron oxide nanoparticles
The invention discloses a preparation method for a high-sensitivity bimodal magnetic resonance contrast agent. According to the preparation method, by means of a method for preparing ferric oleate andmanganese chloride through thermal decomposition, a high-boiling-point solvent is adopted as a reaction medium, oleic acid and oleylamine are used as stabilizers, and therefore manganese oxide embedded iron oxide nanoparticles with narrow particle size distribution and high degree of crystallinity are obtained. The invention particularly relates to a preparation method for modifying the manganeseoxide embedded iron oxide nanoparticles by utilizing the oleic acid / the oleamine, or a preparation method for the biocompatible and water-soluble manganese oxide embedded iron oxide nanoparticles. The preparation method for the high-sensitivity dual-mode magnetic resonance contrast agent has the advantages that the requirements of magnetic resonance imaging for the contrast agent and the characteristics of the Nanotechnology are combined, by means of regulation and control over chemical synthesis, manganese oxide with the T1 contrast capability and superparamagnetic iron oxide nanoparticles with the T2 contrast capability are combined so as to form the manganese oxide embedded iron oxide nanoparticles, and therefore the cooperatively-enhancing dual-mode magnetic resonance contrast effectcan be achieved between the two imaging modes, namely, the T1 imaging mode and the T2 imaging mode.
Owner:BEIJING TECHNOLOGY AND BUSINESS UNIVERSITY
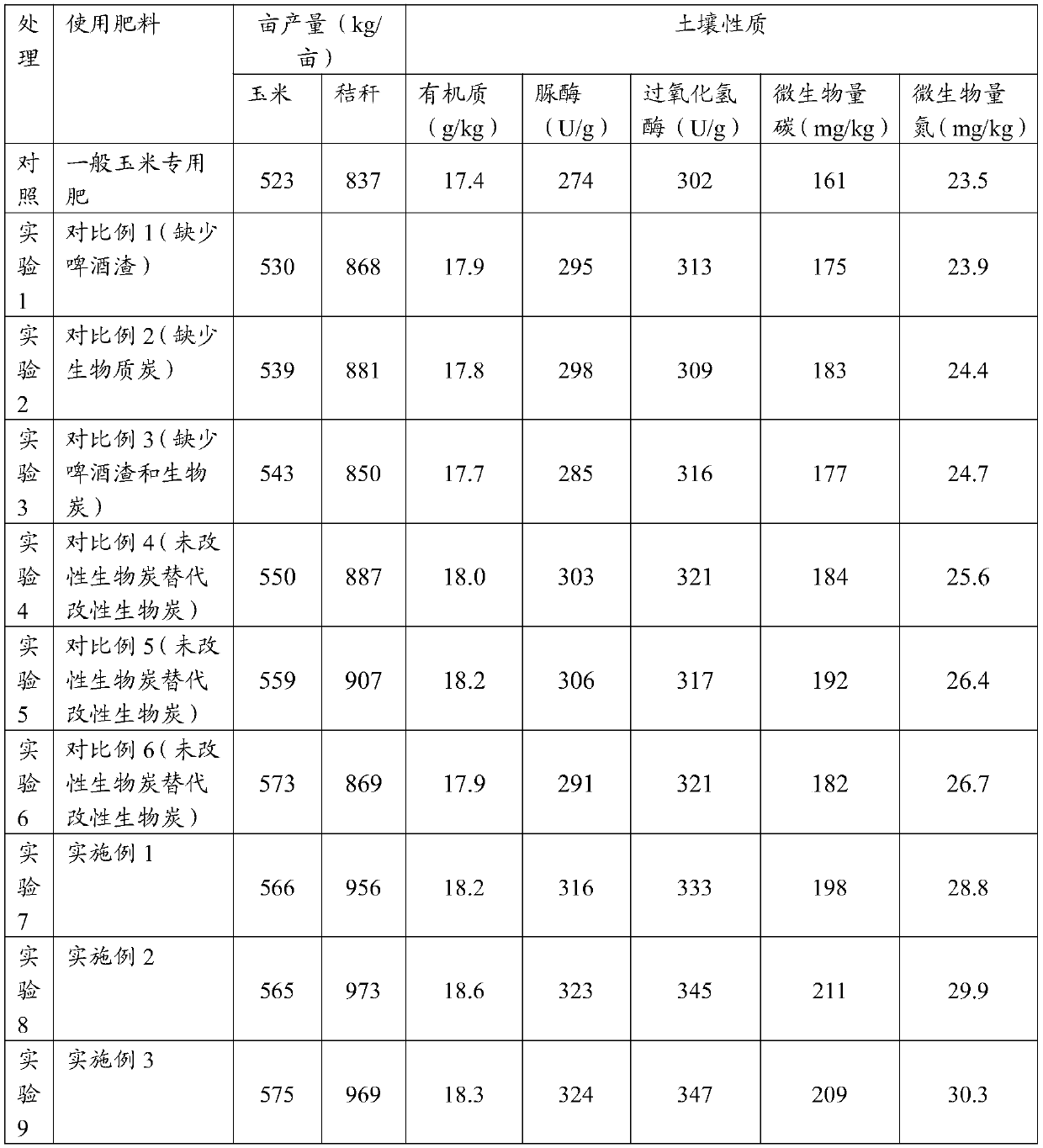
Biochar-based organic fertilizer, preparation method thereof, and application of biochar-based organic fertilizer to crop planting
InactiveCN109879709ASignificant comprehensive benefitsThe generation process is simpleBio-organic fraction processingOrganic fertiliser preparationMicrobial agentOrganic fertilizer
The invention provides biochar-based organic fertilizer, a preparation method thereof, and application of the biochar-based organic fertilizer to crop planting, and belongs to the technical field of fertilizer. The biochar-based organic fertilizer is prepared from the following raw materials in parts by weight: 15-20 parts of graphene / Mn / biochar, 25-40 parts of beer residue, 5-8 parts of zinc andpotassium fulvate, 3-5 parts of beet molasses, 10-20 parts of peanut shell powder, 6-8 parts of an EM microbial agent, 10-30 parts of corn stalk and 50-70 parts of pig manure in a fermentation mode. The preparation method of the graphene / the Mn / the biochar comprises the steps that corn stalk residue powder and the graphene are mixed, heating is conducted for 0.5 h to 2 h at 90 DEG C to 110 DEG C,a product is mixed with a manganese chloride solution, carbonizing is conducted for 3 h to 5 h at 500 DEG C to 600 DEG C, and the graphene / the Mn / the biochar is prepared after ash is removed. The biochar-based organic fertilizer achieves the effects of maintaining the land and increasing production and is an optimal fertilizer source for organic cultivation of corn; and meanwhile, the preparationcost is low, the technology is advanced, and scale production can be achieved.
Owner:QINGDAO AGRI UNIV
Preparation method of carbon-manganese tungsten complex catalyst
InactiveCN107486200AGood dispersionFast degradationMetal/metal-oxides/metal-hydroxide catalystsCarbon layerWater baths
The invention discloses a preparation method of a carbon-manganese tungsten complex catalyst. The preparation method comprises the following steps: adding 5 to 10g of manganese dichloride into 50 to 100mL of water to prepare a solution; then adding 3 to 4g of sodium tungstate into the solution and stirring for 10 to 50 minutes in water bath of 60 to 70 DEG C; then adding 4 to 5g of surfactant Triton X-100 and stirring for 10 to 15 minutes; then adding 1 to 5g of acetone in a continuous stirring process; continuously stirring for 5 to 10 minutes; transferring the solution into a hydrothermal reactor, carrying out reaction for 5 to 10 hours at temperature of 180 to 260 DEG C; carrying out filtration to obtain a solid; washing the solid for 3 to 5 times with distilled water; drying the washed solid at 80 DEG C; putting the dried solid into a tubular furnace; introducing nitrogen gas into the tubular furnace; heating to 700 to 850 DEG C at speed of 10 to 15 DEG C / min and keeping for 30 to 120 minutes; cooling to room temperature under a condition of continuously introducing nitrogen gas so as to obtain the carbon-manganese tungsten complex catalyst. The preparation method of the carbon-manganese tungsten complex catalyst disclosed by the invention has the advantages that by utilizing effects of the nonionic surfactant and acetone, a carbon layer is formed at high temperature and is compounded with manganese tungsten, so that dispersion of manganese tungsten is promoted, and degradation speed of pollutants is improved.
Owner:柳州若思纳米材料科技有限公司 +1
Animal feed additive and preparation method thereof
ActiveCN102550832APromote digestion and absorptionIncrease profitAnimal feeding stuffPotassium hydroxidePotassium iodine
The invention discloses an animal feed additive and a preparation method thereof. The feed additive contains the following components in parts by weight: 15 to 35 parts of table salt, 5 to 15 parts of potassium chloride, 3 to 8 parts of calcium chloride, 0.5 to 1.5 parts of ferrous chloride, 0.3 to 1.5 parts of magnesium, 0.05 to 0.3 part of manganese chloride, 0.2 to 1 part of zinc chloride, 0.05 to 0.3 part of cobalt chloride, 0.01 to 0.05 part of sodium selenite, 0.02 to 0.5 part of potassium iodide, 1 to 5 parts of boric acid, 0.5 to 2 parts of monosilicic acid, 2 to 5 parts of citric acid, 20 to 50 parts of sodium hydroxide, 5 to 10 parts of potassium hydroxide and 150 to 400 parts of water. The method comprises the following steps: dissolving table salt, potassium chloride, calcium chloride, manganese chloride, ferrous chloride, magnesium, zinc chloride, cobalt chloride, sodium selenite and potassium iodide in water; adding boric acid, monosilicic acid and citric acid and allowing acidification chelation reaction to take place for 5 to 10 hours; and adding sodium hydroxide and potassium hydroxide and allowing alkalization reaction to take place for 5 to 10 hours.
Owner:天津市星河系科技有限公司
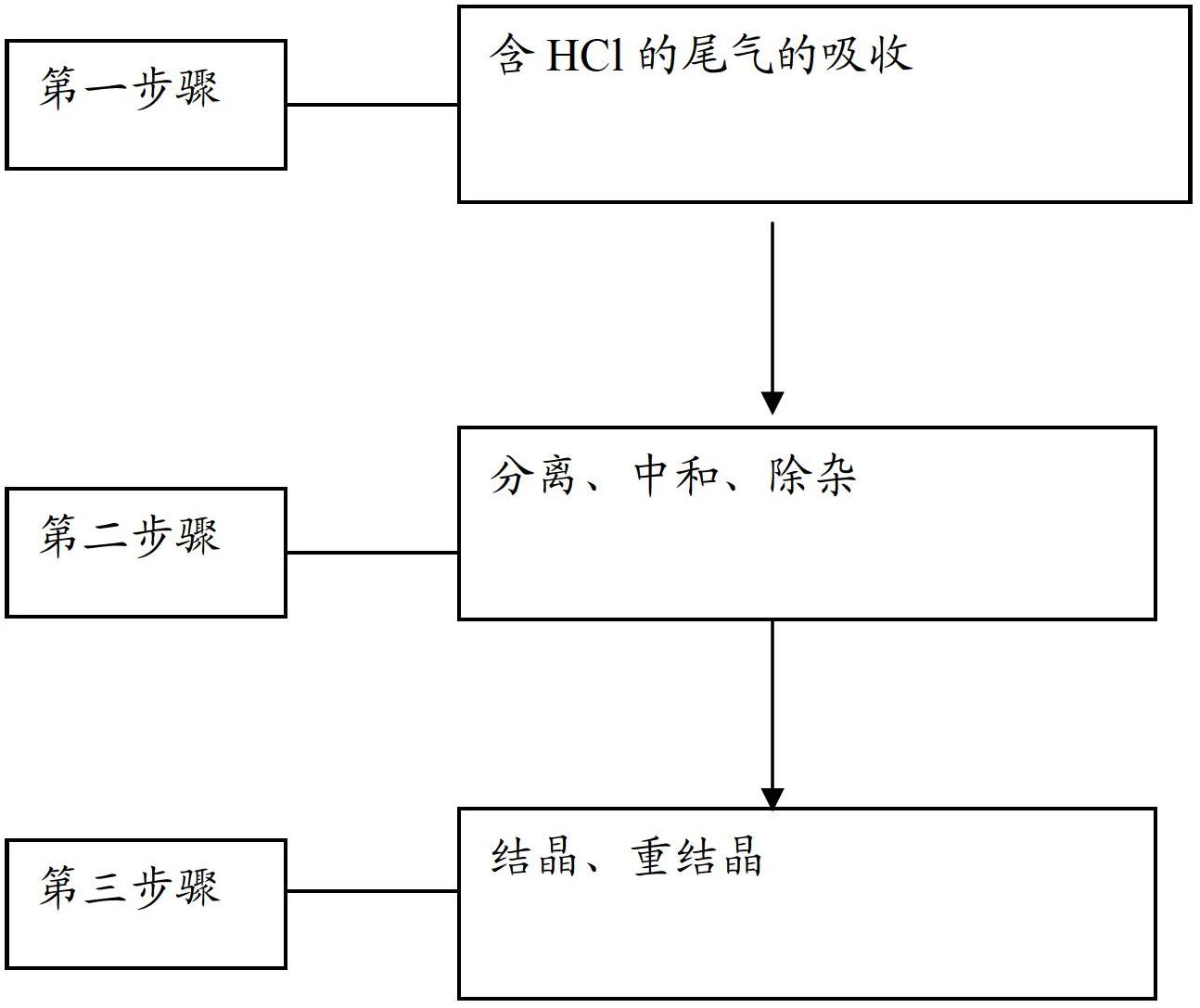
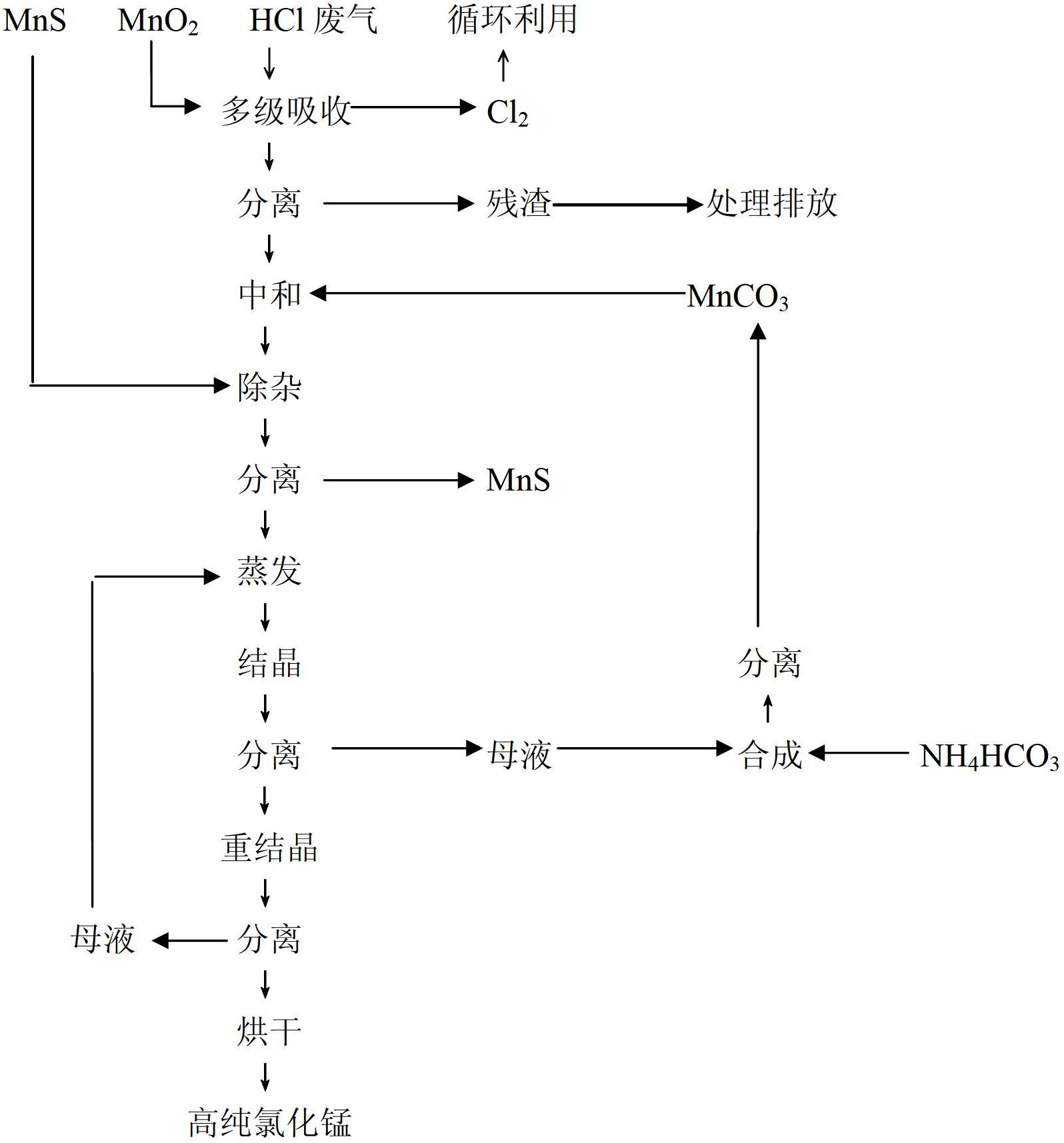
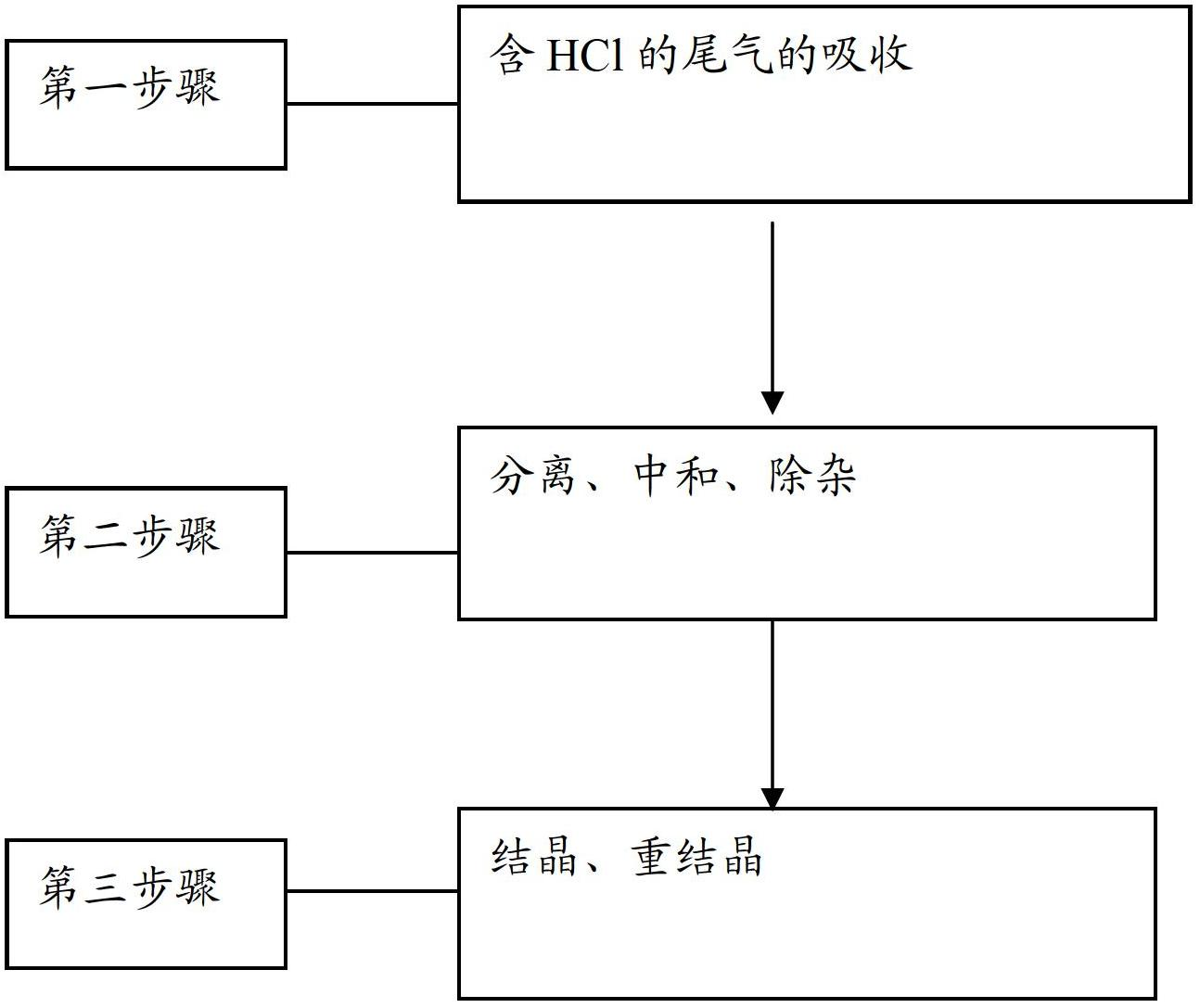
Method for recycling chlorine gas from HCl-containing exhaust gas and preparing manganese chloride, and manganese chloride crystals
InactiveCN102674465ASolve the problem of circular enrichmentSolve highManganese halidesLithiumProduct gas
The invention provides a method for recycling chlorine gas from HCl-containing exhaust gas and preparing manganese chloride, and manganese chloride crystals. The method comprises the following steps: 1. introducing HCl-containing exhaust gas into MnO2 pulp to carry out reaction, stopping the reaction when the pH value of the pulp reaches 1-2, wherein the concentration of MnCl2 in the pulp after the reaction is stopped is controlled at 100-300g / L, and meanwhile, the Cl2-containing gas after the reaction is recycled; 2. carrying out solid-liquid separation on the pulp obtained in the step 1 to obtain a first filtrate, adding manganese-containing alkali liquor into the first filtrate for neutralization until the pH value reaches 3-3.5, removing impurities, and filtering to obtain a second filtrate; and 3. crystallizing the second filtrate, and recrystallizing to obtain the MnCl2.4H2O product. The method can be used for preparing the manganese chloride product which satisfies the following condition required by a lithium ion secondary battery: on the basis of MnCl2.4H2O, the purity is higher than 99.71%.
Owner:GUIZHOU REDSTAR DEVING
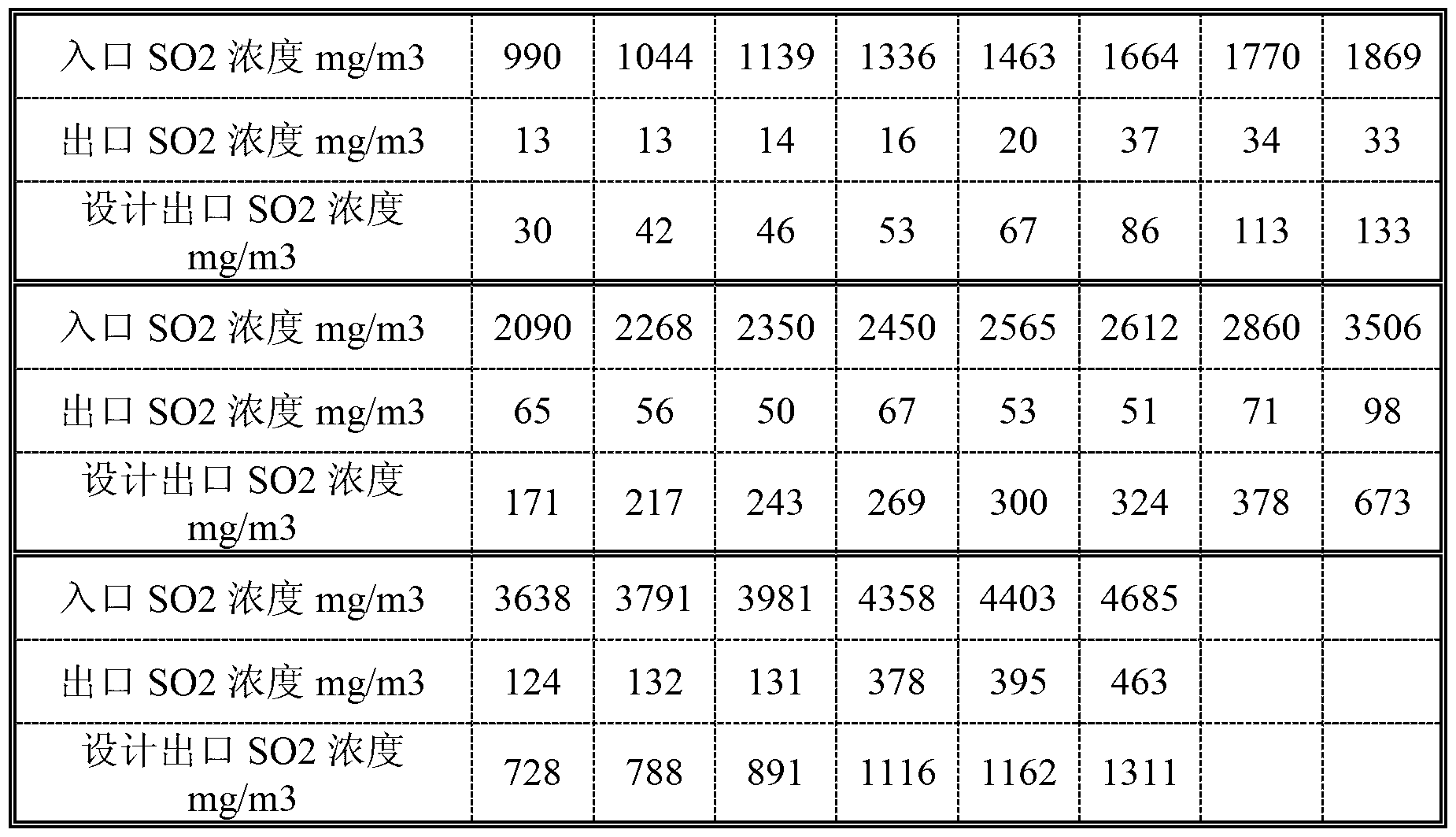
Composite additive for flue gas desulfurization according to limestone method
ActiveCN103071381ASolve poisoningGuaranteed uptimeDispersed particle separationGlutaric acidSalicylic acid
A composite additive for flue gas desulfurization according to a limestone method is composed of the following materials by mass: 85 to 90 percent of nylon acid extracted residue, 1 to 3 percent of salicylic acid, 1 to 3 percent of humic acid, 1 to 5 percent of manganese chloride, and 1 to 5 percent of dimethyl silicone antifoaming agent which are mixed with each other, wherein nylon acid extracted residue is the residue of nylon acid extracted with a large part of glutaric acid. The composite additive has the beneficial effects that the defects of deeper color and unqualified desulfuration drainage chromaticity caused when a nylon acid-type additive is used for flue gas desulfurization are overcome; the pH value buffer zone is wider; sulfur dioxide gas can be dissolved in limestone paste faster; the dissolution of limestone calcium carbonate can be accelerated; the desulfuration efficiency is improved; the cost is greatly reduced; the oxidation efficiency of calcium sulfite is improved; the poisoning problem of limestone paste liquid is solved; foam of a desulfurization tower is eliminated; and the efficient steady operation of the desulfurization tower can be guaranteed.
Owner:天津海得润滋食品有限公司
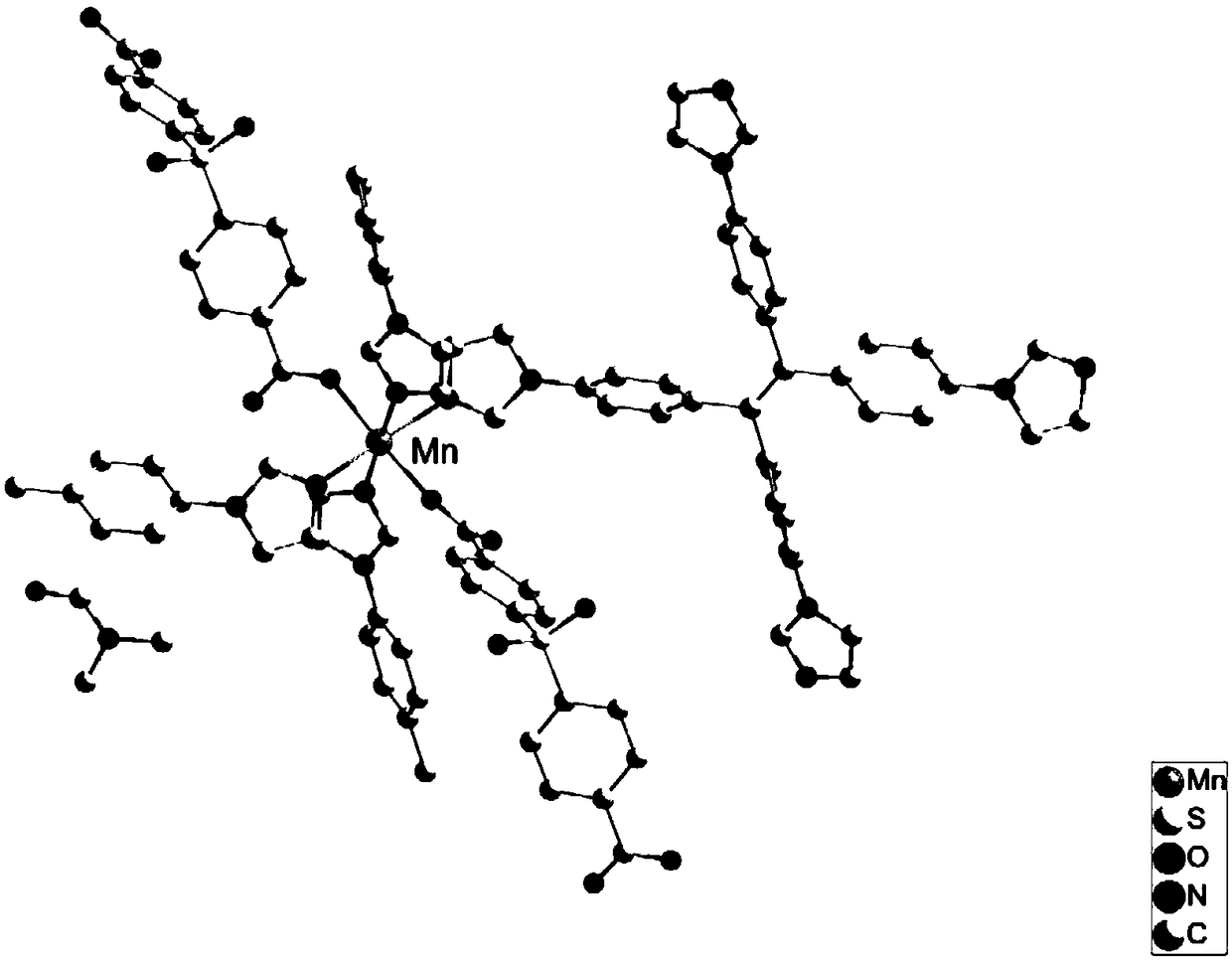
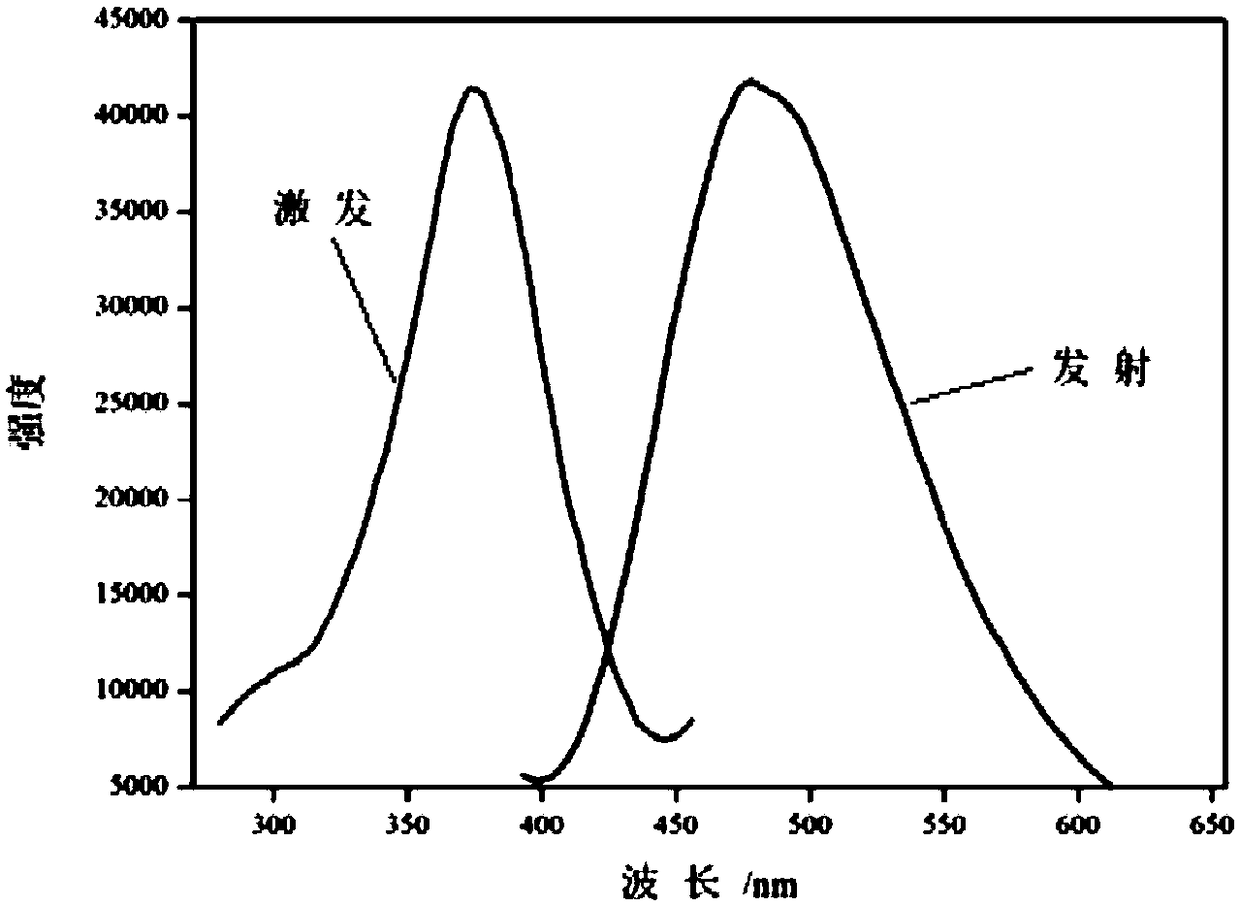
Manganese-based luminescent metal organic framework material as well as preparation method and application thereof
ActiveCN108997433ALimited rotationControl vibrationOrganic chemistry methodsFluorescence/phosphorescenceMetal-organic frameworkSensor materials
The invention discloses a manganese-based luminescent metal organic framework material as well as a preparation method and application thereof. The manganese-based luminescent metal organic frameworkmaterial has a chemical formula of {[Mn(Tipe)4(bcpf)2](DMF)}n. The preparation method comprises the following steps: mixing manganese chloride tetrahydrate, 1,1,2,2-tetra[4-(imidazole-1-yl)phenyl]ethylene and 4,4'-sulfonyl dibenzoic acid according to a ratio, adding N,N-dimethyl formamide and water of a certain volume ratio, mixing and stirring to be filled in a glass bottle, reacting in a constant temperature drying oven at the temperature of 80-100 DEG C for 2-3 days, so as to obtain the colorless product, namely the luminescent metal organic framework material. The luminescent metal organicframework material {[Mn(Tipe)4(bcpf)2](DMF)}n disclosed by the invention can achieve potential application in the detection of a sensor material containing micro water content in the methanol solvent, and has excellent selectivity and high sensitivity.
Owner:JIANGSU UNIV OF SCI & TECH
Preparation method and application of manganese-chromium binary metal oxide energy storage material
ActiveCN108242539ARich reservesLow toxicityCell electrodesSecondary cellsChromium(III) chlorideRetention ratio
The invention relates to a preparation method of a manganese-chromium binary metal oxide energy storage material. The preparation method comprises the following steps: dropwise adding mixed solution of sodium hydroxide and sodium carbonate into mixed solution of manganese chloride and chromium chloride by adopting a coprecipitation method until the mixed solution is alkaline, and then stirring for0.5-2 hours; then ageing for 6-48 hours at the temperature of 25-100 DEG C; washing precipitates, drying and grinding; and then heating and calcining in an aerobic atmosphere, milling, and sieving, thus obtaining the manganese-chromium binary metal oxide energy storage material. The obtained manganese-chromium binary metal oxide energy storage material product is of a nano sheet structure and canalleviate volume expansion effect, inhibit single phase crystal grain agglomeration and shorten a migration path of lithium ions in charging and discharging processes, so that the rate capability ofthe material product is improved, under the condition of 1A / g, from a third cycle, efficiency is higher than 97%, discharge specific capacity after 300 cycles is 913mAh / g, capacity retention ratio is112.0% compared with that of a second circle, and the material product has good application prospect in the aspect of negative electrode materials of a lithium ion battery.
Owner:湖南烯顺新材料有限公司
Renewable high-efficiency formaldehyde removal material and preparation method thereof
InactiveCN107233796ANo secondary pollutionStrong oxidation abilityPhysical/chemical process catalystsDispersed particle separationTetramethylammonium hydroxideMolybdenum carbide
The invention relates to a renewable high-efficiency formaldehyde removal material and a preparation method thereof. The preparation method comprises the following steps of mixing tetramethylammonium hydroxide with a H2O2 solution to make a solution A; dissolving manganese chloride tetrahydrate in ultrapure water to make a solution B; dropwise adding the solution A into the solution B, then ultrasonically dispersing, and agitating to obtain nigger-brown ultrathin MnO2 nanosheet colloidal turbid liquid; dissolving alkaline sepiolite and nano molybdenum carbide in a solution of ethanol and water, dropwise adding the ultrathin MnO2 nanosheet colloidal turbid liquid, centrifugally separating reaction liquid, and drying, so that the high-efficiency formaldehyde removal material loaded with an ultrathin MnO2 nanosheet is obtained. The reaction, of the renewable high-efficiency formaldehyde removal material, of catalytically oxidizing formaldehyde can be carried out at room temperature, is excellent in recycling performance, is an environment-friendly and nontoxic powdery catalyst, can be used for effectively removing the formaldehyde released from various house furnishing materials and free formaldehyde in air, and is thoroughly free from secondary pollution; thus, obsession and threat which are brought by the formaldehyde in a living environment to the normal life of people are guaranteed to remove.
Owner:XIAN LOW CARBON ENERGY SAVING TECH SERVICECO +2
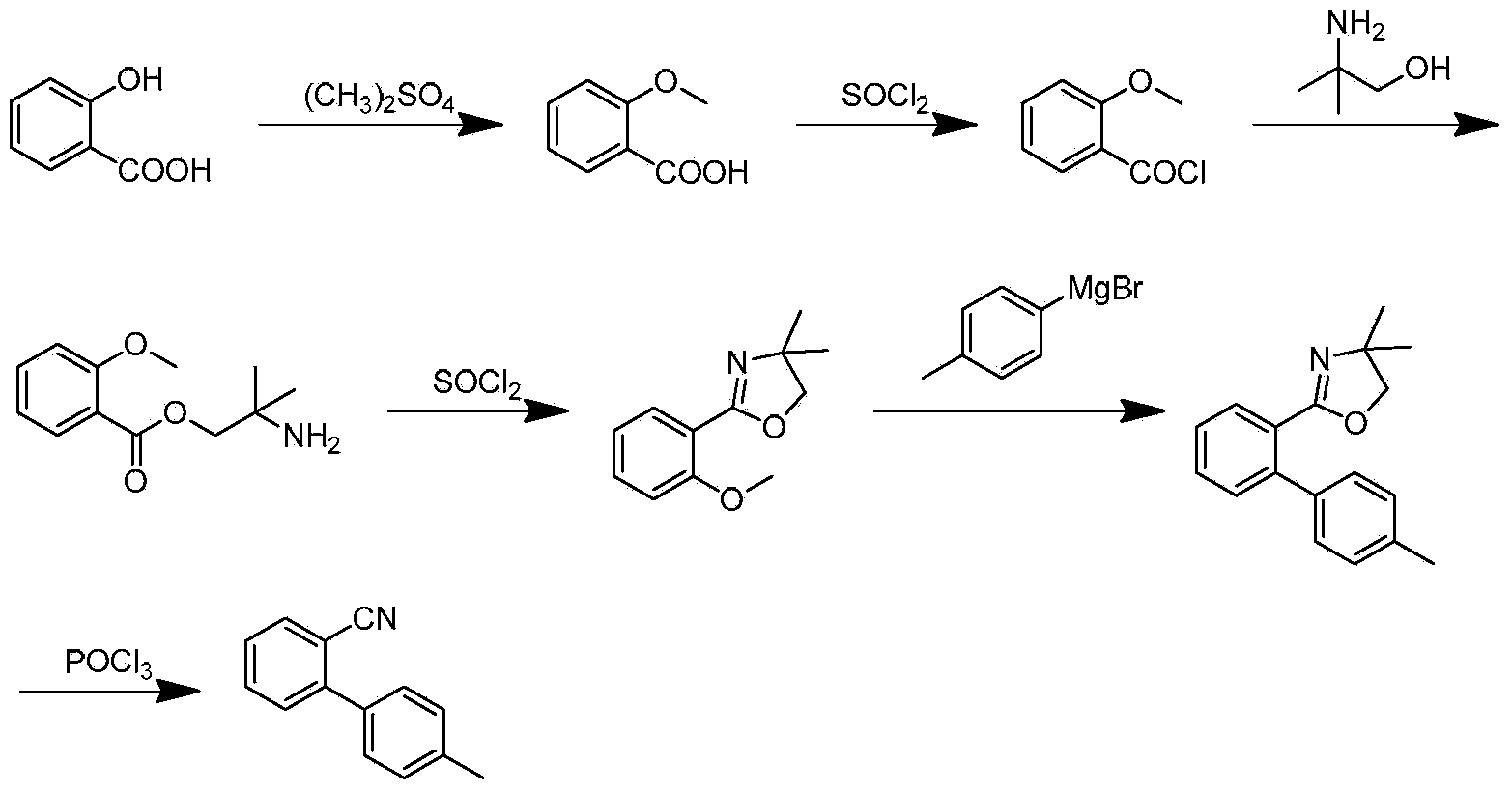
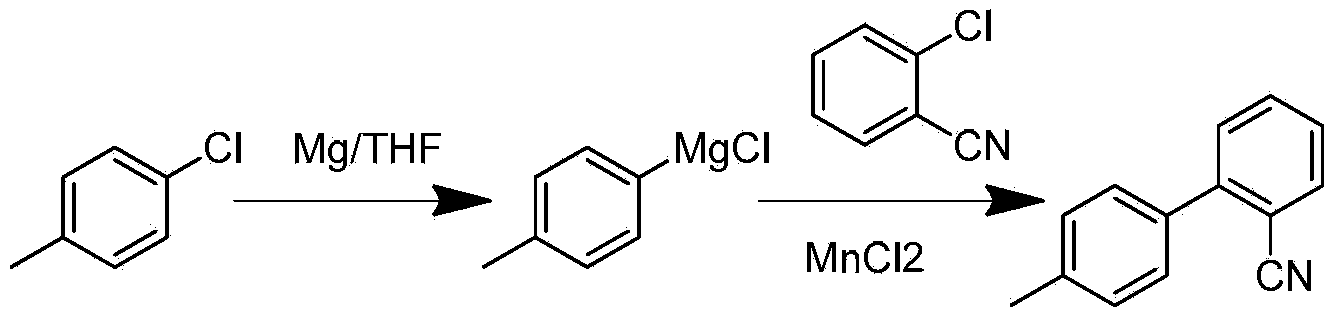
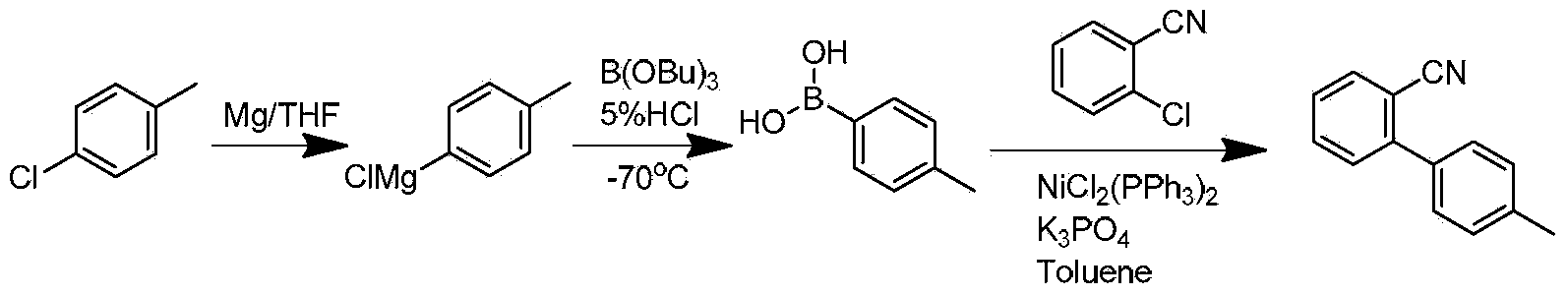
Preparation method for 2- cyanogroup-4 '-methyl diphenyl
ActiveCN104072387AReduce usageReduce concentrationCarboxylic acid nitrile preparationOrganic compound preparationGrignard reagentCoupling reaction
The invention discloses a preparation method for 2- cyanogroup-4 '-methyl diphenyl. The preparation method comprises the following steps: (1), reacting in tetrahydrofuran in the presence of an initiator by using magnesium powder and parachlorotoluene to obtain a p-methylphenyl magnesium chloride Grignard reagent; (2), under catalysis of manganese chloride, dropwise adding the p-methylphenyl magnesium chloride Grignard reagent into tetrahydrofuran liquor of chlorobenzonitrile to carry out coupling reaction, treating part of reaction liquor to obtain 2- cyanogroup-4 '-methyl diphenyl; and (3), under catalysis of manganese chloride, by taking residual reaction liquor in the step (2) as a backing material, adding the tetrahydrofuran liquor of chlorobenzonitrile, dropwise adding the p-methylphenyl magnesium chloride Grignard reagent to carry out coupling reaction to obtain the 2- cyanogroup-4 '-methyl diphenyl. The preparation method disclosed by the invention strengthens catalytic efficiency of coupling reaction by using part of the reaction liquor, improves reaction conversion rate, reduces production of byproduct dimethyl biphenyl, and is suitable for industrial production.
Owner:SHANDONG HANXING PHARM TECH CO LTD +1
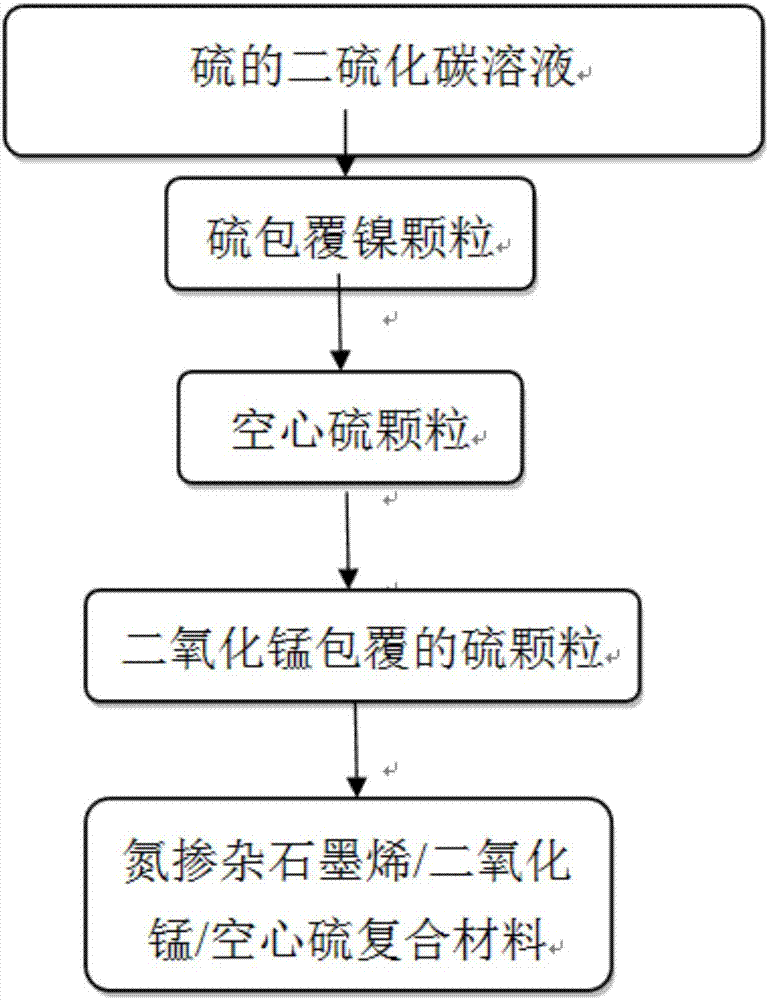
Preparation method for nitrogen doped graphene/manganese dioxide/hollow sulfur composite material
ActiveCN106941161AImprove conductivityImprove electrochemical performanceMaterial nanotechnologyCell electrodesFiltrationHigh energy
The invention provides a preparation method for a nitrogen doped graphene / manganese dioxide / hollow sulfur composite material, wherein the preparation method comprises the following multiple steps: step (1), adding a sulfur powder to carbon disulfide, and stirring and dissolving to form a homogeneous solution; step (2), carrying out ball milling of a high-purity nickel powder by a high energy ball mill, after ball milling, adding into the solution, stirring to form a homogeneous suspension, mechanically stirring, and spray-drying to form sulfur-coated spherical particles; step (3) adding the spherical particles to a ferric chloride solution, carrying out stirring reaction, washing with water, and filtering; and step (4), adding the precipitate after filtration to a solution containing manganese chloride and potassium permanganate, stirring into a homogeneous suspension, carrying out heating and stirring reaction, centrifuging, and washing with water to obtain sulfur particles coated with manganese dioxide. The design of a hollow structure of the composite material can reserve a space for volume expansion of the sulfur material in charging and discharging processes, and the electrochemical performance can be effectively improved.
Owner:常熟东南高新技术创业服务有限公司
Method for synthesizing MnS micrometer powder with controllable morphology
The invention discloses a method for synthesizing MnS micrometer powder with a controllable morphology and relates to the technical field of function energy materials. The method comprises the following steps of: dissolving manganese chloride tetrahydrate and a sulfur source in water, uniformly stirring, transferring the well-dissolved solution into a high-temperature and high-pressure reaction kettle lined with polytetrafluoroethylene, then placing in a constant-temperature blowing oven to carry out reaction, and after the reaction is complete, carrying out aftertreatment to obtain the MnS micrometer powder; the sulfur source is sodium sulfate, L-cysteine or sodium thiosulfate. The MnS micrometer powder with the controllable morphology is successively obtained by a solvent method. Shown by a series of experimental results, when Na2S.9H2O is used as the sulfur source, the product is a sheet-shaped octahedron; when Na2S2O3.5H2O is used as the sulfur source, the product is a bipyramid octahedron; when LC3H7NO2S is used as the sulfur source, the product is small-rod-shaped; the obtained product is uniform in grain size and good in dispersity and has no agglomeration.
Owner:HEFEI UNIV
Manganese doped inorganic halogen perovskite quantum dots and preparation method thereof
ActiveCN108728089AReduce manufacturing costImprove production efficiencyMaterial nanotechnologyNanoopticsQuantum yieldHalogen
The invention discloses manganese doped inorganic halogen perovskite quantum dots and a preparation method thereof. The preparation method comprises the following steps: lead salt and manganese chloride are dissolved and heated at 80-110 DEG C, and a halogen precursor is obtained; cesium salt is dissolved, and a cesium precursor is obtained; the cesium precursor is added to the halogen precursor,the mixture is heated at 80-110 DEG C, and the manganese doped inorganic halogen perovskite quantum dots are obtained. The method for preparing the manganese doped inorganic halogen perovskite quantumdots with high yield at lower temperature needs no inert gas protection, preparation cost is reduced, preparation efficiency is improved, quantum yield is as high as 62.41% which is 12.41% higher than the current highest quantum yield of 54% with a hot injection method, and the method can be applied to mass production.
Owner:HUAZHONG UNIV OF SCI & TECH
Modified silica gel carrier and supported metallocene catalyst, preparation methods thereof as well as metallocene catalyst system
The invention belongs to the field of olefin polymerization catalysts and particularly relates to a modified silica gel carrier and a supported metallocene catalyst, preparation methods thereof as well as a metallocene catalyst system. The modified silica gel carrier comprises the following reaction products: silica gel, manganese chloride and alkylaluminoxane; alkylaluminoxane has the general formula as shown in the specification, wherein R is selected from C1-C12 alkyls; and a is an integer from 4 to 30, and preferably, an integer from 10 to 30. The preparation method of the modified silica gel carrier is simple, and the prepared catalyst is good in particle morphology; in addition, the supported metallocene catalyst is wide in application range in term of an olefin polymerization process and high in polymerization activity, the obtained polymers are high in stacking density, and the molecular weights and distribution of the polymers are basically kept constant.
Owner:CHINA PETROLEUM & CHEM CORP +1
Compound synergistic additive for wet-process flue gas desulphurization and preparation method of compound synergistic additive for wet-process flue gas desulphurization
InactiveCN105664702AEasy to prepareLow costGas treatmentDispersed particle separationHydrogenGas phase
The invention discloses a compound synergistic additive for wet-process flue gas desulphurization and a preparation method of the compound synergistic additive for wet-process flue gas desulphurization.The compound synergistic additive comprises, by weight, 55-80 parts of adipic acid, 5-15 parts of nylon acid, 5-14 parts of sodium citrate, 1-10 parts of sodium sulfate, 3-10 parts of ferric sulfate and 2-10 parts of manganese chloride.The preparation method of the compound synergistic additive includes: at the normal temperature and under normal pressure, weighing the adipic acid, the nylon acid, the sodium citrate, the sodium sulfate, the ferric sulfate and the manganese chloride according to the parts by weight while mixing and stirring uniformly.The compound synergistic additive has the advantages that the compound synergistic additive is simple to prepare and low in cost; limestone activity is promoted, calcium carbonate dissolution speed is increased and limestone consumption and gas-phase and liquid-phase resistance are reduced, so that desulphurization efficiency is improved; calcium sulphite oxidation rate can be promoted, pH (potential of hydrogen) buffer ability of slurry is improved and CaSO3 mild scale generation is inhibited to protect running equipment.
Owner:武汉光谷环保科技股份有限公司 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com