Method for synthesizing MnS micrometer powder with controllable morphology
A synthesis method, micron powder technology, applied in chemical instruments and methods, manganese compounds, inorganic chemistry, etc., can solve the problems that have not been reported in the literature, and achieve the effect of low cost, dispersion without agglomeration, and uniform particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
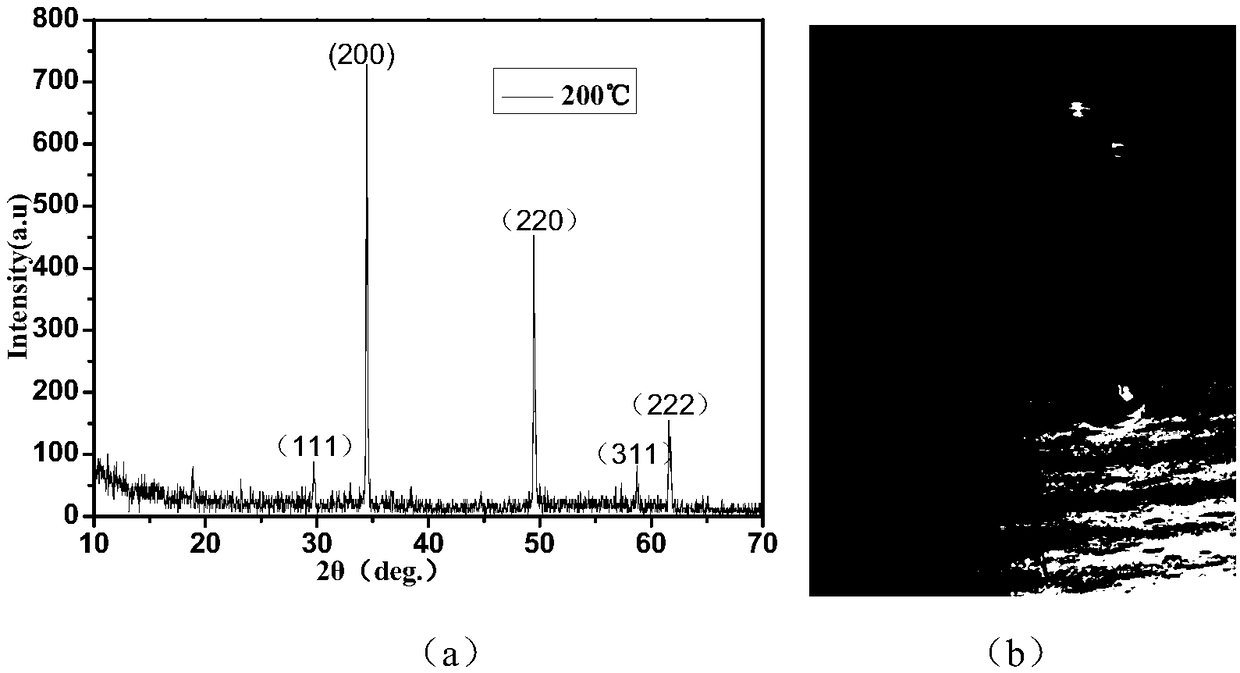
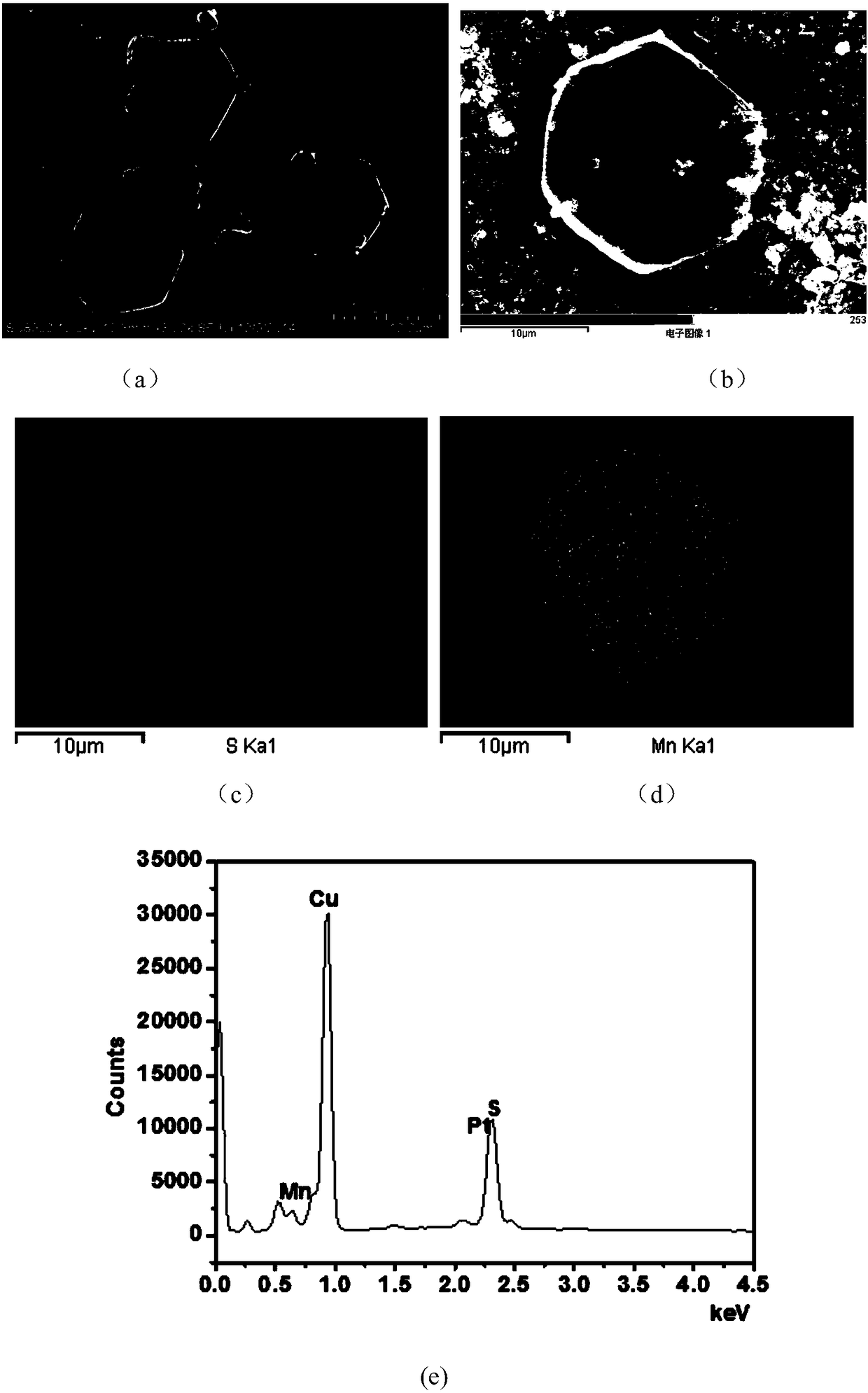
[0022] (1) Weigh 1mmol of MnCl with an electronic balance at room temperature 2 4H 2 O, weigh 2 mmol of Na 2 S·9H 2 0. Transfer the weighed medicine into the beaker, measure 10mL of deionized water with a graduated cylinder and add it to the beaker, put it into a magnet, and place the beaker on a magnetic stirrer to stir for 20min.
[0023] (2) Transfer the dissolved solution to a polytetrafluoroethylene-lined high-temperature and high-pressure reaction kettle, tighten the lid of the reaction kettle, and put the high-temperature and high-pressure reaction kettle into an electric heating constant temperature blast drying oven to heat, and the temperature is set at 200°C , The heating time is 24h.
[0024] (3) After the reaction is finished, wait for the reaction kettle to cool down to room temperature and then turn off the power supply of the electric heating constant temperature blast drying oven, and then take the reaction kettle out of the electric heating constant temper...
Embodiment 2
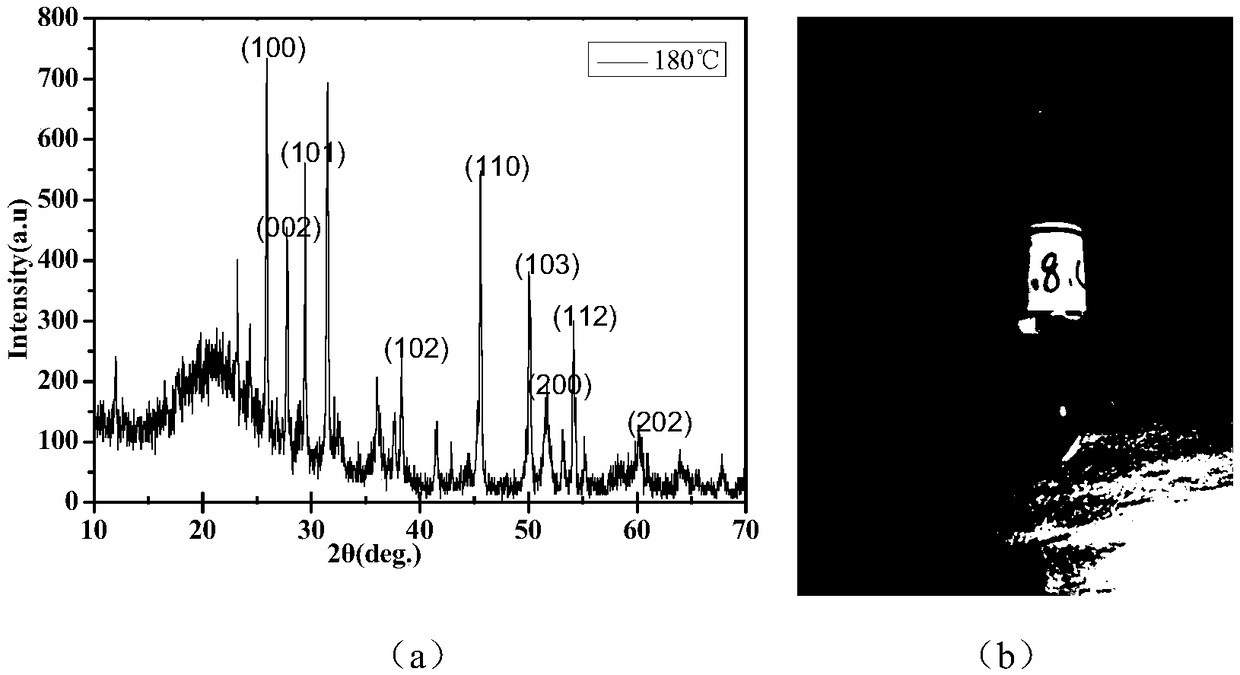
[0029] (1) Weigh 1mmol of MnCl with an electronic balance at room temperature 2 4H 2 O, weigh 4 mmol of Na 2 S·9H 2 0, transfer the weighed medicine into the beaker, measure 8mL of deionized water with a graduated cylinder and add it to the beaker, put into the magnet, and place the beaker on a magnetic stirrer to stir for 20min.
[0030] (2) Transfer the dissolved solution to a polytetrafluoroethylene-lined high-temperature and high-pressure reaction kettle, tighten the lid of the reaction kettle, and put the high-temperature and high-pressure reaction kettle into an electric heating constant temperature blast drying oven to heat, and the temperature is set at 180°C , The heating time is 10h.
[0031] (3) After the reaction is finished, wait for the reaction kettle to cool down to room temperature and then turn off the power supply of the electric heating constant temperature blast drying oven, and then take the reaction kettle out of the electric heating constant temperat...
Embodiment 3
[0036] (1) Weigh 1mmol of MnCl with an electronic balance at room temperature 2 4H 2 O, weigh 6 mmol of Na 2 S·9H 2 0. Transfer the weighed medicine into the beaker, measure 10mL of deionized water with a graduated cylinder and add it to the beaker, put it into a magnet, and place the beaker on a magnetic stirrer to stir for 20min.
[0037] (2) Transfer the dissolved solution to a polytetrafluoroethylene-lined high-temperature and high-pressure reaction kettle, tighten the lid of the reaction kettle, and put the high-temperature and high-pressure reaction kettle into an electric constant temperature blast drying oven for heating, and set the temperature to 160°C , The heating time is 18h.
[0038] (3) After the reaction is finished, wait for the reaction kettle to cool down to room temperature and then turn off the power supply of the electric heating constant temperature blast drying oven, and then take the reaction kettle out of the electric heating constant temperature b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com