Method for preparing battery-grade manganese sulfate by using copper chloride manganese liquid
A technology of manganese sulfate and copper chloride, applied in the direction of manganese sulfate and the like, can solve the problems of high cost of extraction and decalcification, difficult process control, etc., and achieve the effects of reducing burden, saving costs, and increasing the concentration of manganese ions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
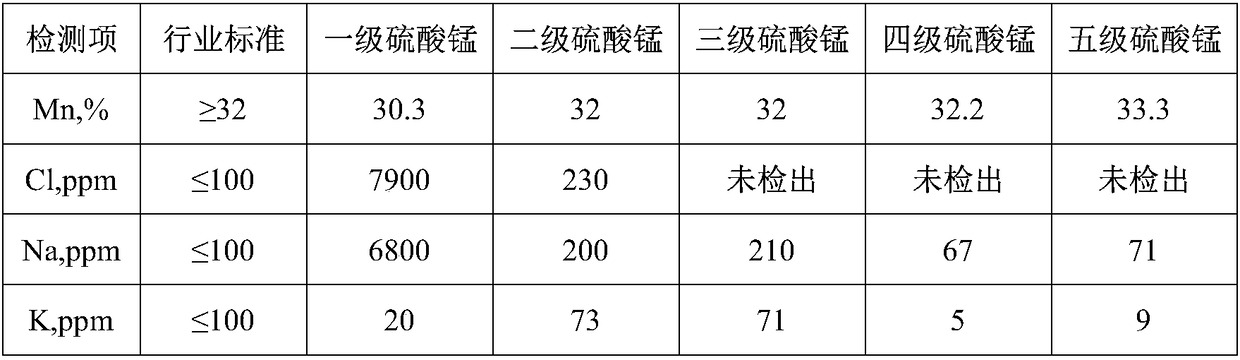
[0044] Copper manganese chloride solution is taken from a well-known domestic enterprise group, and its chemical composition is (g / L): Mn 129, Cu 1.633, Zn6.711, Co 2.795, Ca 10.2, Al 2.274, H + 0.65mol / L.
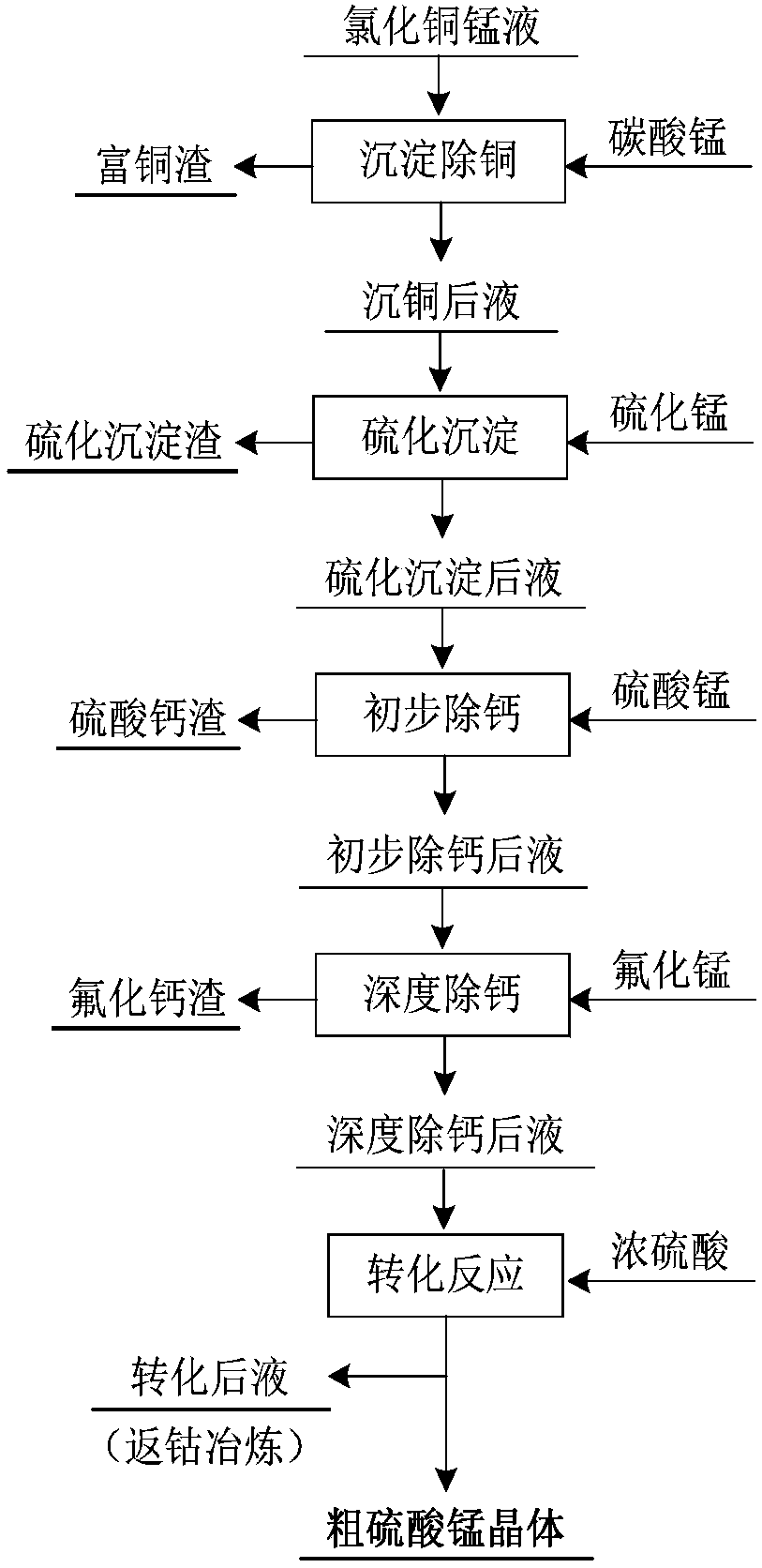
[0045] Step 1: manganese carbonate adjusts the pH value of the solution to selectively precipitate copper: add manganese carbonate powder to the copper manganese chloride solution, wherein the molar ratio of manganese carbonate powder to hydrogen ions in the solution is 0.43:1.0, slowly add in batches, and control the pH of the system The value is 3.0, the reaction temperature is 60° C., and the reaction time is 3.0 h. After the reaction, solid-liquid separation is carried out to obtain the copper-removed liquid and copper-rich slag (basic copper chloride).
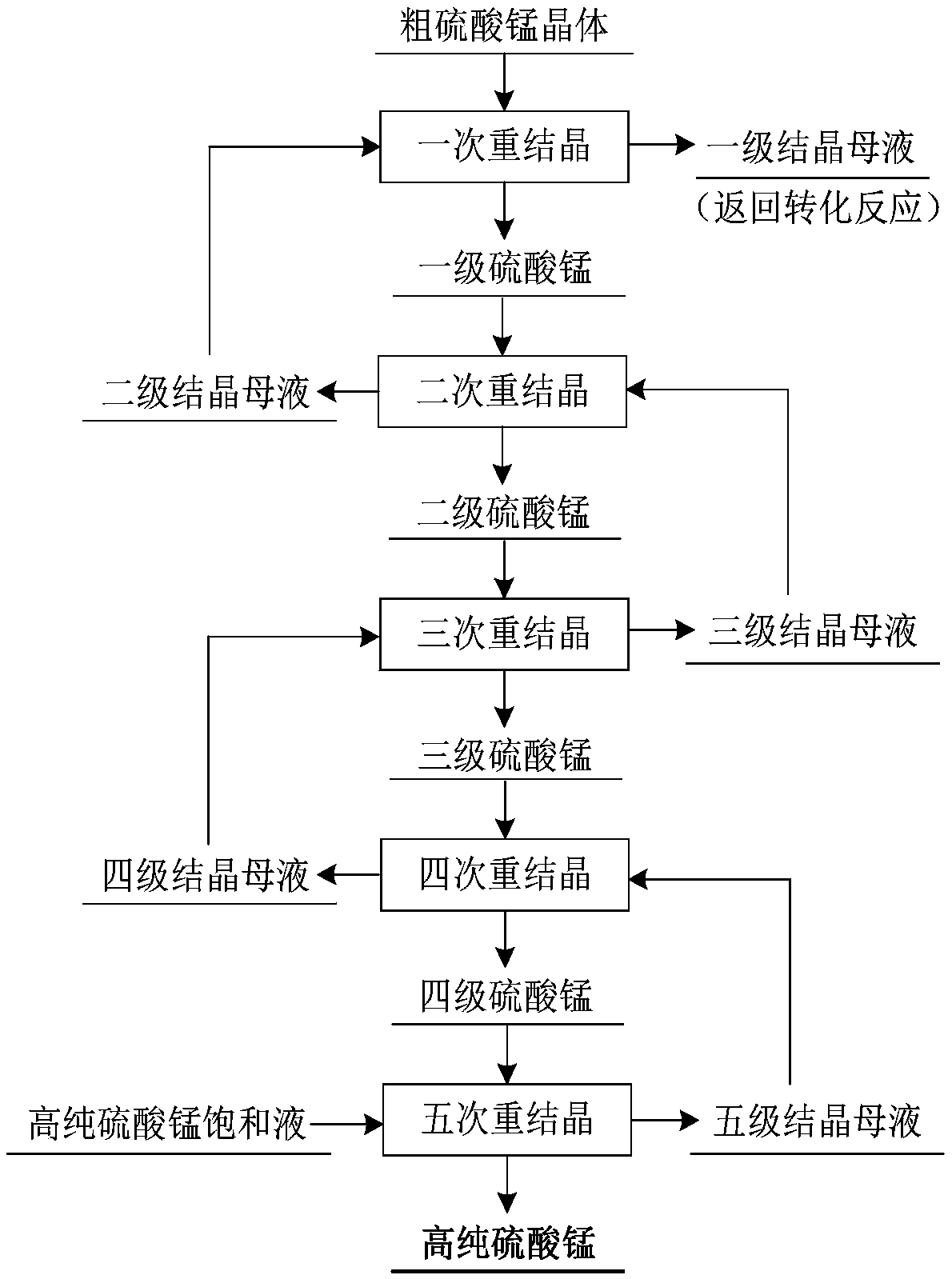
[0046] Step 2: Add manganese sulfide powder according to the ratio of the amount of Me (Me represents the sum of Cu, Co, Zn) and MnS in the liquid after copper precipitation is 1.0:1.1, and heat the reaction system t...
Embodiment 2
[0055] Copper manganese chloride solution is taken from a well-known domestic enterprise group, and its chemical composition is (g / L): Mn 120, Cu 0.833, Zn5.24, Co 1.41, Ca 5.2, Al 2.25, H + 0.25mol / L.
[0056] Step 1: Manganese carbonate adjusts the pH value of the solution to selectively precipitate copper: add manganese carbonate powder to the copper manganese chloride solution, wherein the molar ratio of manganese carbonate powder to hydrogen ions in the solution is 0.35:1.0, slowly add in batches, and control the pH of the system The value is 2.8, the reaction temperature is 55°C, and the reaction time is 3.5h. After the reaction, solid-liquid separation is carried out to obtain the copper-removed liquid and copper-rich slag (basic copper chloride).
[0057] Step 2: Add manganese sulfide powder according to the ratio of the amount of Me (Me represents the sum of Cu, Co, Zn) and MnS in the liquid after copper precipitation is 1.0:1.2, and heat the reaction system to 90°C ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com