Synthesis method and application method of manganese Prussian blue analog material for lithium ion battery
A technology similar to Prussian blue and lithium-ion batteries, which is applied in the synthesis and application of manganese-based Prussian blue materials for lithium-ion batteries. It can solve the problems of limited theoretical capacity and poor cycle stability of graphite materials, and achieve improved electrochemical performance. Reduced reaction time and reduced production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1: Preparation method and structural characterization of manganese-based Prussian blue-like materials for lithium-ion batteries
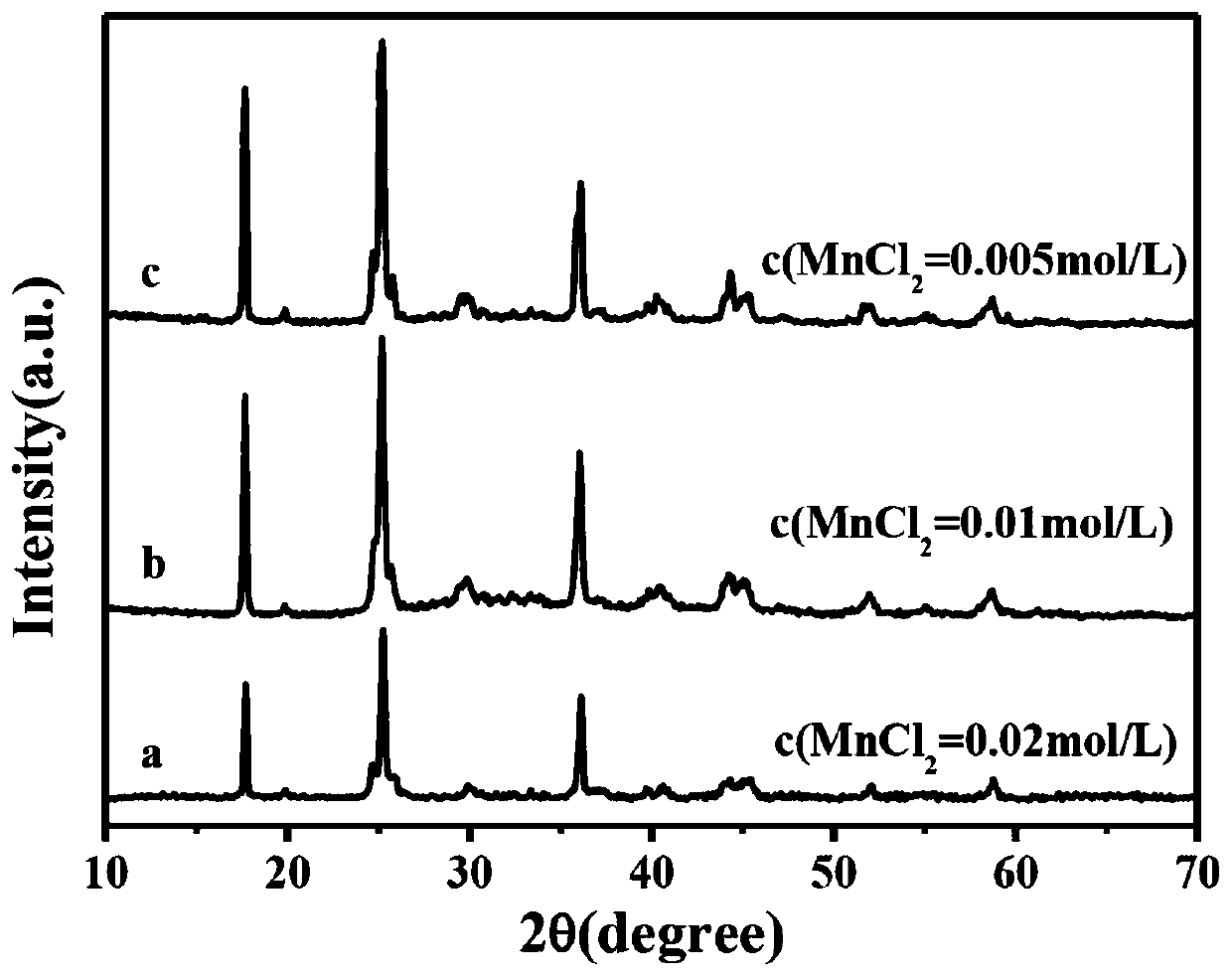
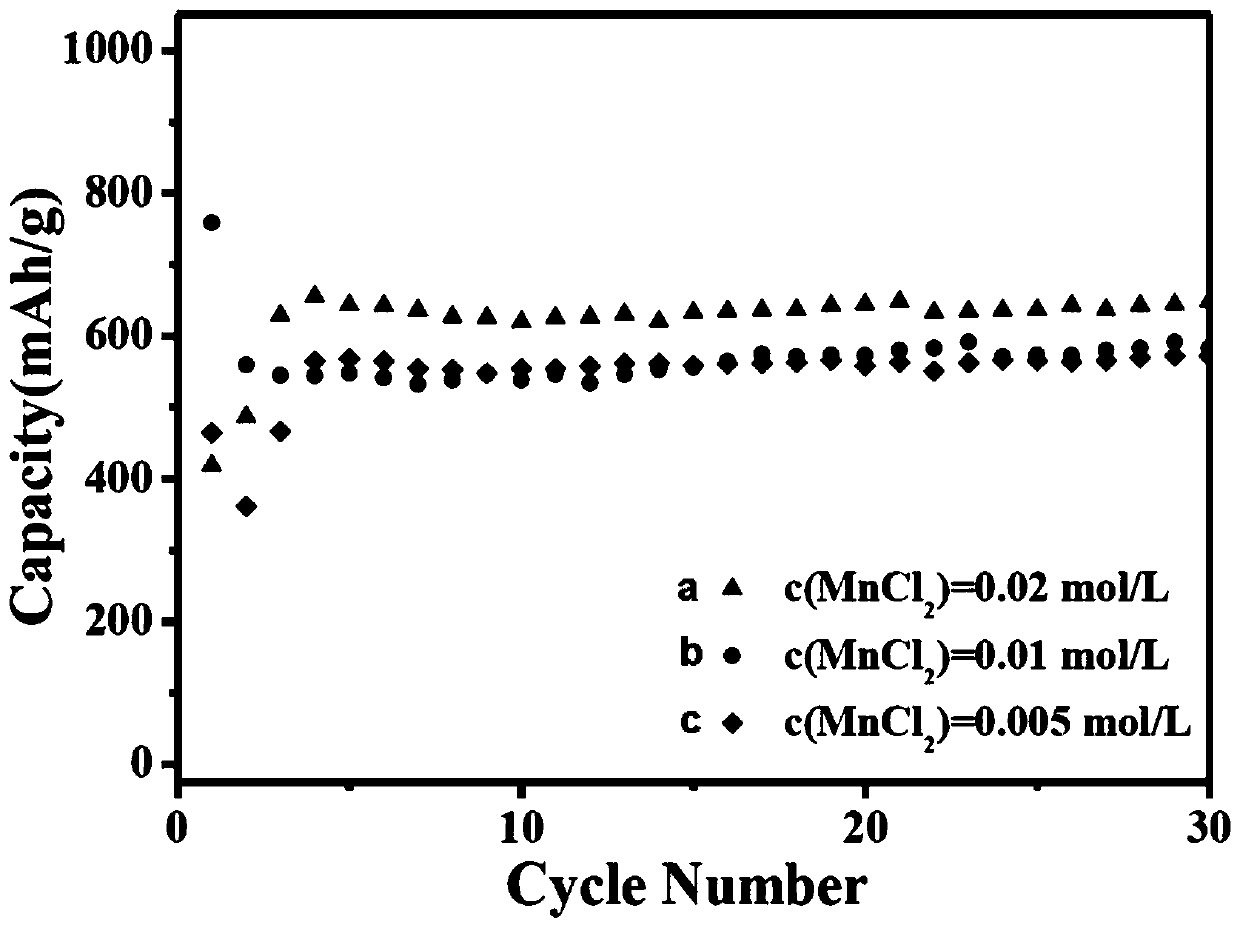
[0054] The source of manganese is anhydrous manganese chloride (MnCl 2 ), ferricyanide comes from potassium ferricyanide (K 3 [Fe(CN) 6 ]), the chelate selects sodium citrate dihydrate;
[0055] Weigh three 1mmol anhydrous manganese chloride (MnCl 2 ) and 2mmol sodium citrate dihydrate were completely dissolved in 25ml, 50ml, and 100ml deionized water respectively to obtain colorless, clear and transparent solution A, solution B and solution C respectively. Another weighed 1mmol potassium ferricyanide (K 3 [Fe(CN) 6 ]) was dissolved in 25ml of deionized water to obtain solution D. After stirring and dissolving completely, pour C part of solution D into solution A, solution B and solution C respectively to obtain solution E, solution F and solution G respectively, and stand still at room temperature After 12 hours, they were coll...
Embodiment 2
[0062] Embodiment 2: weigh 1mmol anhydrous manganese chloride (MnCl 2 ), 2mmol sodium citrate dihydrate was completely dissolved in 25ml deionized water to obtain a colorless, clear and transparent solution A. Weigh again 1mmol potassium ferricyanide (K 3 [Fe(CN) 6 ]) was dissolved in 25ml of deionized water to obtain solution B, and solution B was poured into solution A and mixed evenly to obtain solution C, which was left to stand at room temperature for 6 hours, separated and purified, and dried at 60°C for 12 to 24 hours to obtain white powder product. The product is manganese-like Prussian blue, the microscopic shape is cubic, and the microscopic particle size is 150-180nm.
Embodiment 3
[0063] Embodiment 3: weigh 0.5mmol manganese chloride monohydrate (MnCl 2 ·H 2 (0), 2mmol anhydrous sodium citrate was completely dissolved in 25ml deionized water to obtain a colorless, clear and transparent solution A. Weigh again 1mmol potassium ferricyanide (K 3 [Fe(CN) 6 ]) was dissolved in 25ml deionized water to obtain solution B, and solution B was poured into solution A and mixed uniformly to obtain solution C, which was left to stand at room temperature for 24 hours to obtain the final white powder product. The product is a manganese-like Prussian blue with a cubic microscopic shape and a particle size distribution of 310-410nm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com