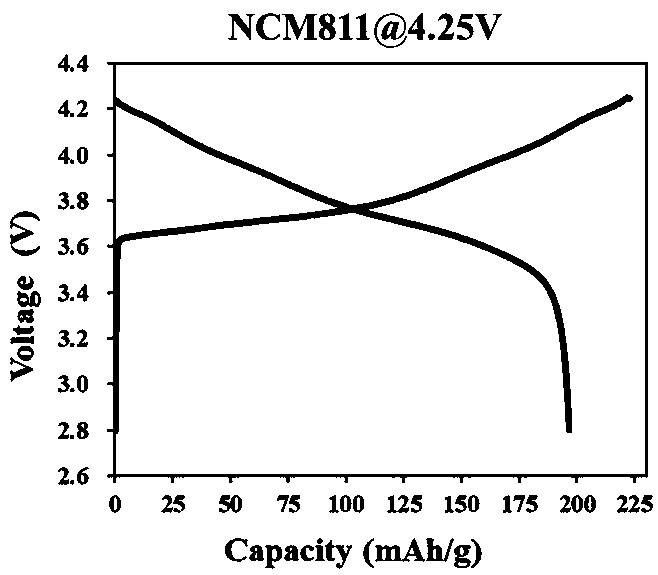
Preparation method of ternary positive electrode material of lithium-rich manganese-based coating layer
A positive electrode material, lithium-rich manganese-based technology, applied in the field of new energy material preparation, can solve the problems of capacity drop, impedance rise, etc., and achieve the effects of improved cycle stability, stable material performance, and excellent electrochemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] NiSO 4 , CoSO 4 , Mn 2 (SO 4 ) 3 According to the ratio of Ni:Co:Mn=7:1.5:1.5, the mixed aqueous solution of nickel, cobalt and manganese was prepared with a concentration of 1.5 mol / L, and the reactor was used for co-precipitation reaction, and an appropriate amount of saturated ammonia and KOH were added to adjust the pH value. Subsequently, the solution and 2M KOH aqueous solution were fed into the reaction kettle separately. During the reaction, keep the pH value at 11, NH 4 OH is added as a chelating agent. Adjust the reaction rate to ensure that the average residence time of the reaction is 4-12 hours. The coprecipitate was filtered, washed and dried at 120°C for 24 hours to obtain Ni 0.7 co 0.15 mn 0.15 (OH) 2 Precursor. Mixed with LiOH at a molar ratio of 1:1.03, preheated at 500°C for 5h, and calcined at 800°C for 15h to obtain LiNi0.7Co0.15Mn0.15O2. The prepared ternary positive electrode material was mixed with lithium nitrate, nickel nitrate, man...
Embodiment 2
[0028] NiSO 4 , CoSO 4 , Mn 2 (SO 4 ) 3 The mixed aqueous solution of nickel, cobalt and manganese was prepared according to the molar ratio Ni: Co: Mn = 8: 1: 1, the concentration was 1 mol / L, and the reactor was used for co-precipitation reaction, and an appropriate amount of saturated ammonia and NaOH were added to adjust the pH value. Subsequently, the solution and 2M NaOH aqueous solution were fed into the reaction kettle separately. During the reaction, keep the pH value at 11, NH 4 OH is added as a chelating agent. Adjust the reaction rate to ensure that the average residence time of the reaction is 4-12 hours. The coprecipitate was filtered, washed and dried at 120°C for 24 hours to obtain Ni 0.7 co 0.15 mn 0.15 (OH) 2 Precursor. Mix with LiOH at a molar ratio of 1:1.03, preheat at 500°C for 5h, and calcinate at 800°C for 15h to obtain LiNi 0.8 co 0.1 mn 0.1 o 2 . The prepared ternary positive electrode material was mixed with lithium nitrate, nickel ni...
Embodiment 3
[0030] NiSO 4, CoSO 4 , Mn 2 (SO 4 ) 3 The mixed aqueous solution of nickel, cobalt and manganese was prepared according to the molar ratio Ni: Co: Mn = 8: 1: 1, the concentration was 1 mol / L, and the reactor was used for co-precipitation reaction, and an appropriate amount of saturated ammonia and NaOH were added to adjust the pH value. Subsequently, the solution and 2M NaOH aqueous solution were fed into the reaction kettle separately. During the reaction, keep the pH value at 11, NH 4 OH is added as a chelating agent. Adjust the reaction rate to ensure that the average residence time of the reaction is 4-12 hours. The coprecipitate was filtered, washed and dried at 120°C for 24 hours to obtain Ni 0.7 co 0.15 mn 0.15 (OH) 2 Precursor. Mixed with LiOH at a molar ratio of 1:1.03, preheated at 450°C for 5h, and calcined at 820°C for 15h to obtain LiNi 0.8 co 0.1 mn 0.1 o 2 . The prepared ternary positive electrode material was mixed with lithium nitrate, nickel ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com