Positive electrode material for lithium ion battery and preparation method of positive electrode material
A technology for lithium-ion batteries and cathode materials, applied in battery electrodes, secondary batteries, circuits, etc., can solve the problems of large irreversible capacity loss, reduced capacity on discharge platform, poor cycle stability, etc., and achieve excellent rate performance and energy density. Loss mitigation, effect of stabilizing circulation ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
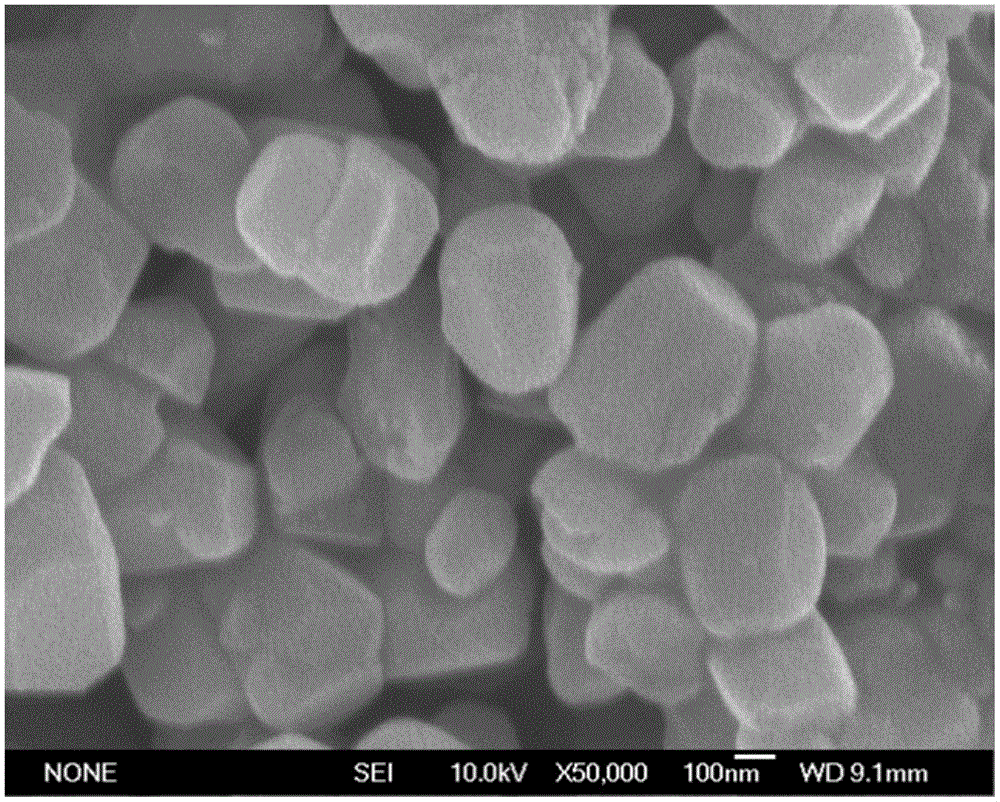
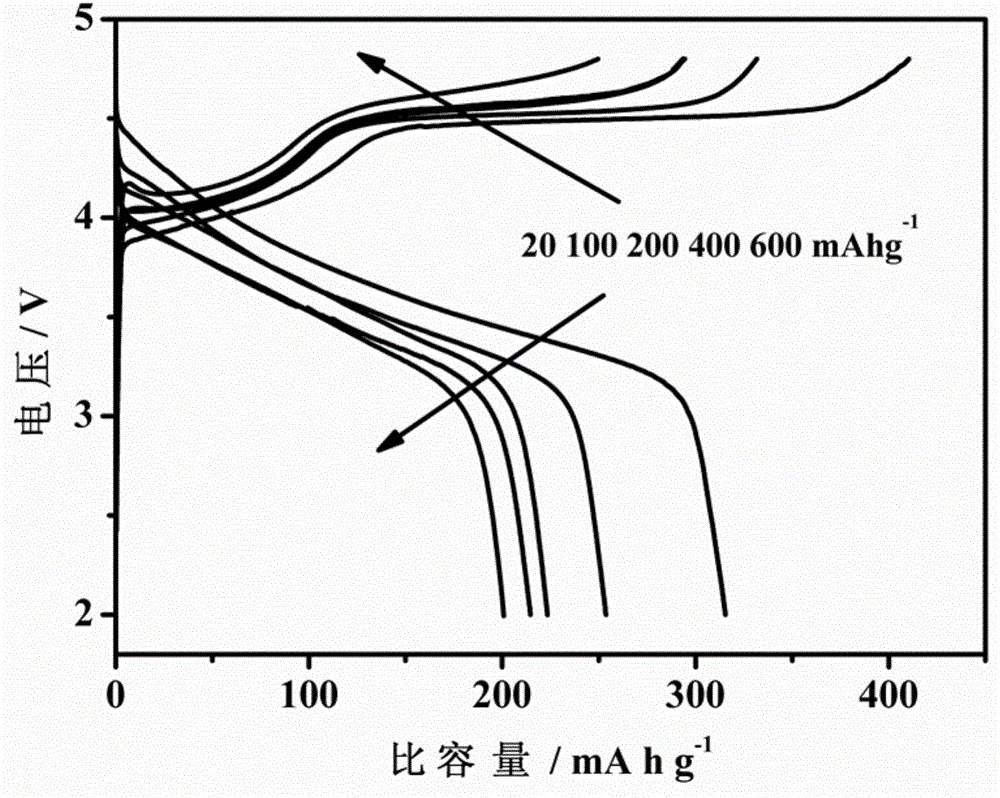
Image
Examples
Embodiment 1
[0044] Using potassium permanganate, manganese sulfate, and concentrated sulfuric acid as starting materials, dissolve 0.474g of potassium permanganate, 0.845g of manganese sulfate, and 1mL of concentrated sulfuric acid in 15mL of deionized water, stir for 10min to form a homogeneous solution, and transfer it to In the reaction kettle, heat up to 150°C and keep it for 20 minutes. After cooling down, filter with suction, wash with deionized water for 3 times, and then dry to obtain a potassium-doped manganese precursor; mix 0.55g of the obtained precursor with 0.4430g of nickel nitrate, 0.4440g of cobalt nitrate After fully grinding and mixing with 0.5909g lithium hydroxide, put it into a crucible; heat up to 900°C in a box furnace at a heating rate of 2°C / min and heat for 12h, and cool at room temperature to obtain x=0.62 Cathode material Li (3+x) / 3-y K y mn (1+x) / 3 co (1-x) / 3 Ni (1-x) / 3 o 2 , where y=(1+x) / 162, recorded as sample 1#.
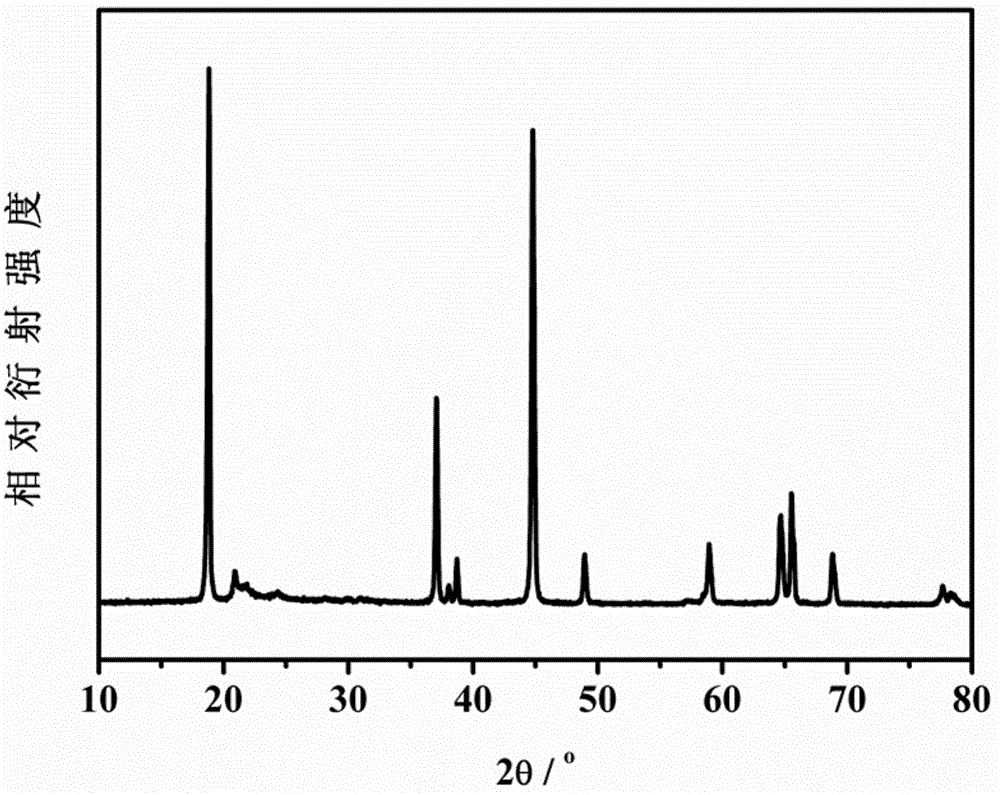
[0045] The XRD test results of sam...
Embodiment 2
[0049] Using potassium permanganate, manganese sulfate, and concentrated sulfuric acid as starting materials, dissolve 0.474g of potassium permanganate, 0.845g of manganese sulfate, and 1mL of concentrated sulfuric acid in 15mL of deionized water, stir for 10min to form a homogeneous solution, and transfer it to In the reaction kettle, the temperature was raised to 160°C and kept for 30 minutes. After cooling down, it was filtered with suction, washed with deionized water for 3 times, and then dried to obtain a potassium-doped manganese precursor; After fully grinding and mixing with 0.6690g lithium hydroxide, put it into a crucible; heat up to 850°C in a box furnace at a heating rate of 2°C / min and heat for 12h, and cool in ice water to obtain x=0.3 Cathode material Li (3+x) / 3-y K y mn (1+x) / 3 co (1-x) / 3 Ni (1-x) / 3 o 2 , where y=(1+x) / 162, recorded as sample 2#.
[0050] The XRD test results of sample 2# show that its XRD diffraction peak position is the same as that of...
Embodiment 3
[0054] Using potassium permanganate, manganese sulfate, and concentrated sulfuric acid as starting materials, dissolve 0.474g of potassium permanganate, 0.845g of manganese sulfate, and 1mL of concentrated sulfuric acid in 15mL of deionized water, stir for 10min to form a homogeneous solution, and transfer it to In the reaction kettle, the temperature was raised to 150°C for 20 minutes, the temperature was lowered, and then suction filtered, washed with deionized water for 3 times, and then dried to obtain a potassium-doped manganese precursor; 1.0 g of the obtained precursor was mixed with 0.1744 g of nickel nitrate, 0.1745 g of cobalt nitrate After fully grinding and mixing with 0.9820g lithium hydroxide, put it into a crucible; heat up to 950°C in a box furnace at a heating rate of 2°C / min for 12 hours, and cool in liquid nitrogen to obtain x=0.9 The cathode material Li (3+x) / 3-y K y mn (1+x) / 3 co (1-x) / 3 Ni (1-x) / 3 o 2 , where y=(1+x) / 162, recorded as sample 3#.
[0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com