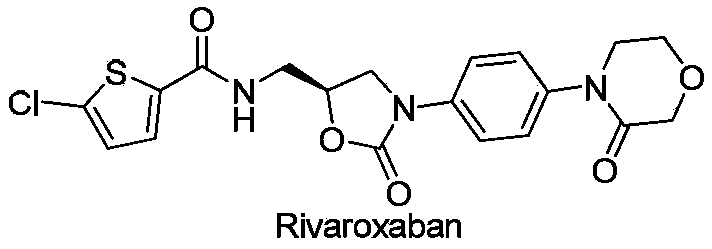
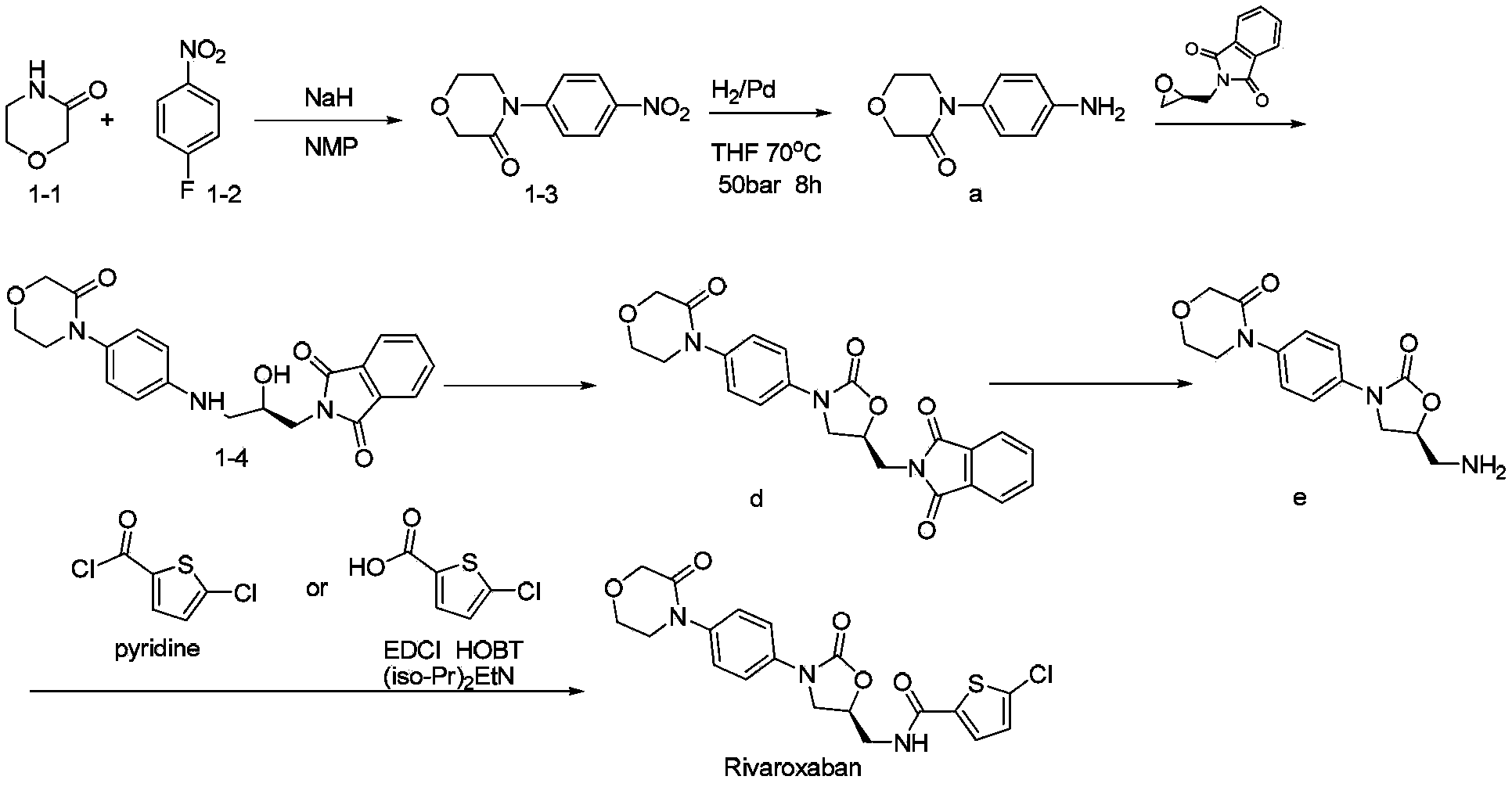
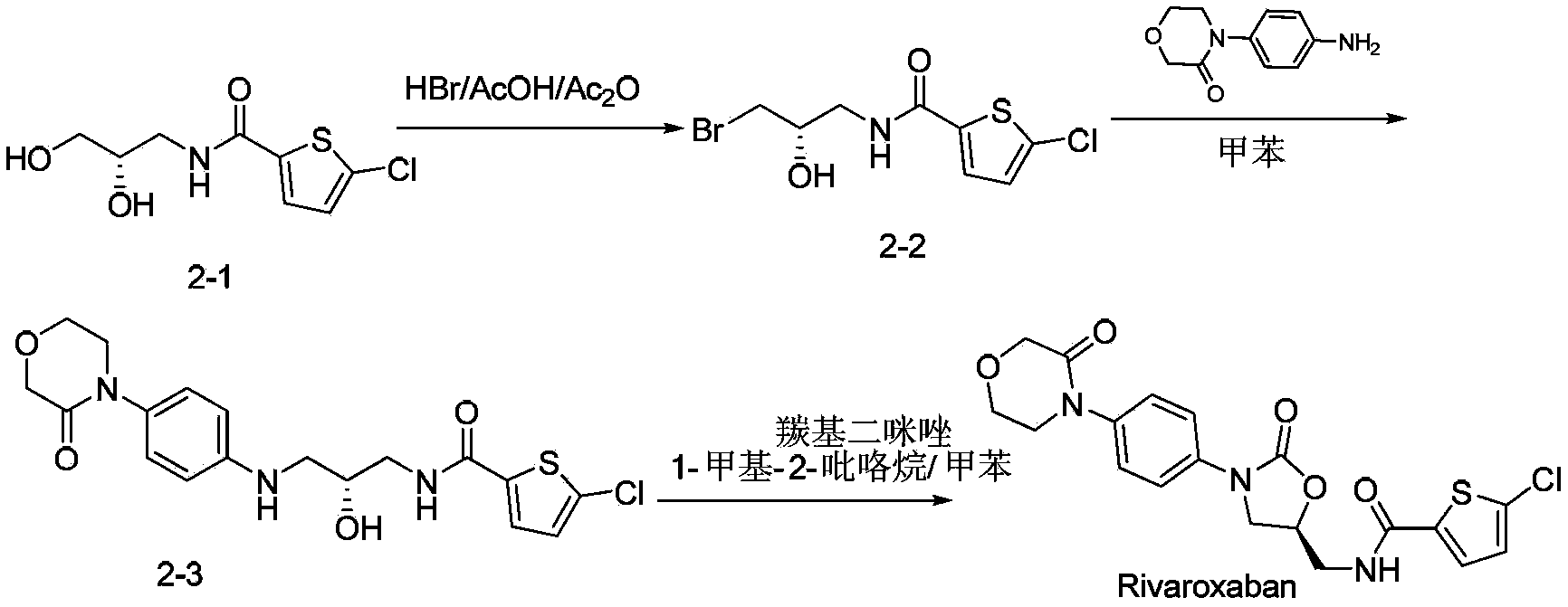
Novel synthetic process for rivaroxaban
A compound, nitrophenyl technology, applied in the field of heterocyclic chemistry and nitrogen-oxygen heterocyclic chemistry, can solve the problems of difficulty in separation and purification, difficult industrialization, and high toxicity of reagents, and achieves high reaction yield, less by-products, easy to use. The effect of purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Embodiment 1: the preparation of compound Ia
[0072]
[0073] Compound VIa (3.30 g, 19 mmol), compound VII (4.15 g, 9 mmol), and 20 ml of N-methylpyrrolidone were sequentially added into a 100 ml single-neck round bottom flask, and magnetic stirring was started. Add anhydrous lithium bromide (0.5g, 5.8mmol) and tri-n-butylphosphine oxide (0.5g, 2.3mmol), heat up to 90-95°C, and keep the reaction for 11 hours. After the reaction is complete, cool down to 30°C, add 50ml of dichloromethane and 30ml of water, stir for 5 minutes, and filter. The filtrate was separated into layers, the lower organic phase was removed, and the upper aqueous phase was discarded. The organic phase was washed with 30ml×3 water, concentrated to dryness under reduced pressure, and the residue was subjected to column chromatography (300-400 mesh silica gel). Obtained) 3.3 g of the compound of formula Ia as a white solid, with a yield of 93.8%.
Embodiment 2
[0074] Embodiment 2: the preparation of compound Ib
[0075]
[0076] Add compound VIb (25.0 g, 120.6 mmol), compound VII (20.8 g, 95.3 mmol), and 200 ml of N,N-dimethylformamide to a 250 ml single-neck round bottom flask in sequence, and start magnetic stirring. Add 1.5g of anhydrous lithium bromide and 2.0g of tri-n-butylphosphine oxide, heat up to 80-85°C, and keep it warm for 10 hours. After the reaction, the temperature was lowered to 30°C, 500ml of dichloromethane and 300ml of water were added, stirred for 10 minutes, and filtered. The filtrate was separated into layers, the lower organic phase was removed, and the upper aqueous phase was discarded. The organic phase was washed with 300ml×3 water, concentrated to dryness under reduced pressure, and the residue was subjected to column chromatography (200-300 mesh silica gel). Obtained) 36.9 g (77.3 mmol) of the compound of formula Ib as a white solid, with a yield of 91.1% and an HPLC purity of 99.3%.
[0077] 1 H ...
Embodiment 3
[0078] Embodiment 3: the preparation of compound Ic
[0079]
[0080] Compound VIc (15.0 g, 84.7 mmol), compound VII (13.2 g, 60.5 mmol), and 150 ml of N-methylpyrrolidone were sequentially added into a 250 ml single-neck round bottom flask, and mechanical stirring was started. Add 0.9 g of anhydrous lithium bromide and 1.0 g of tri-n-butylphosphine oxide, heat up to 90-95° C., and keep the reaction for 7 hours. After the reaction is complete, cool down to 30°C, add 400ml of dichloromethane and 250ml of water, stir for 10 minutes, and filter. The filtrate was separated into layers, the lower organic phase was removed, and the upper aqueous phase was discarded. The organic phase was washed with 100ml×3 water, concentrated to dryness under reduced pressure, and the residue was subjected to column chromatography (200-300 mesh silica gel). Obtained) 22.1 g (49.8 mmol) of the compound of formula Ic as a white solid, with a yield of 92.5% and an HPLC purity of 98.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com