O-cumaric acid LEuH complex and synthesis method thereof
A synthesis method, o-coumaric acid technology, applied in chemical instruments and methods, fluorescence/phosphorescence, luminescent materials, etc., can solve the problems of large error and low detection sensitivity, and achieve the effect of improving sensitivity and enhancing fluorescence performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] The synthetic method of above-mentioned o-coumaric acid-LEuH complex can comprise the following steps:
[0039] 1) Synthesis of LEuH:
[0040] Eu(NO 3 ) 3 ·6H 2 O, NaNO 3 , Hexamethylenetetramine was dissolved in water to obtain an aqueous solution, in which Eu(NO 3 ) 3 ·6H 2 O, NaNO 3 , the molar ratio of hexamethylenetetramine is: 1: (10-15): (0.5-1.5); preferably Eu(NO3) 3 ·6H 2 O, NaNO 3 1. Hexamethylenetetramine is dissolved in degassed water, and degassed water refers to the water that got rid of the dissolved gas in the water, which can be obtained by boiling distilled water for 10 minutes, and the degassed water amount is preferably 60-120ml / mmolEu(NO 3 ) 3 ·6H 2 O.
[0041] N was introduced into the above aqueous solution 2 , into N 2 The time is preferably 3-10 minutes, and then the hydrothermal reaction is carried out. The preferred reaction temperature is 70-100°C; the preferred reaction time is 12-18h. After the reaction is completed, filter ...
Embodiment 1
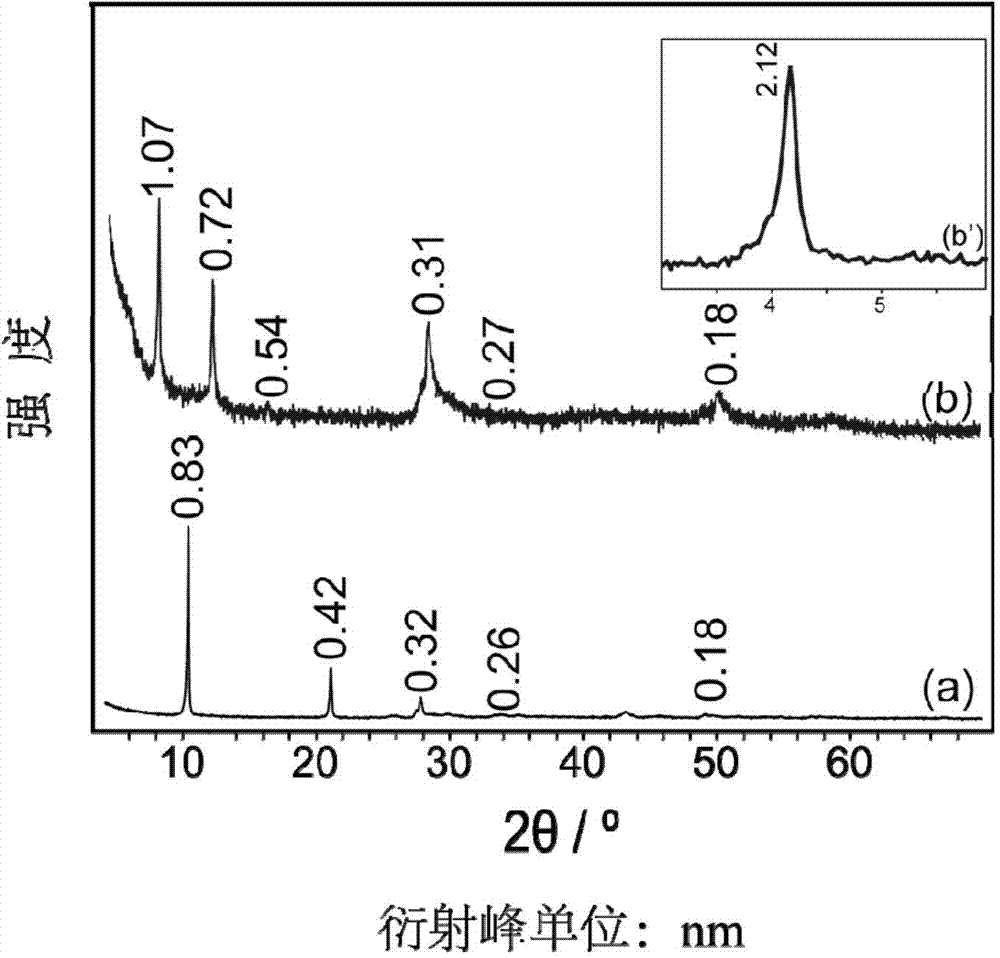
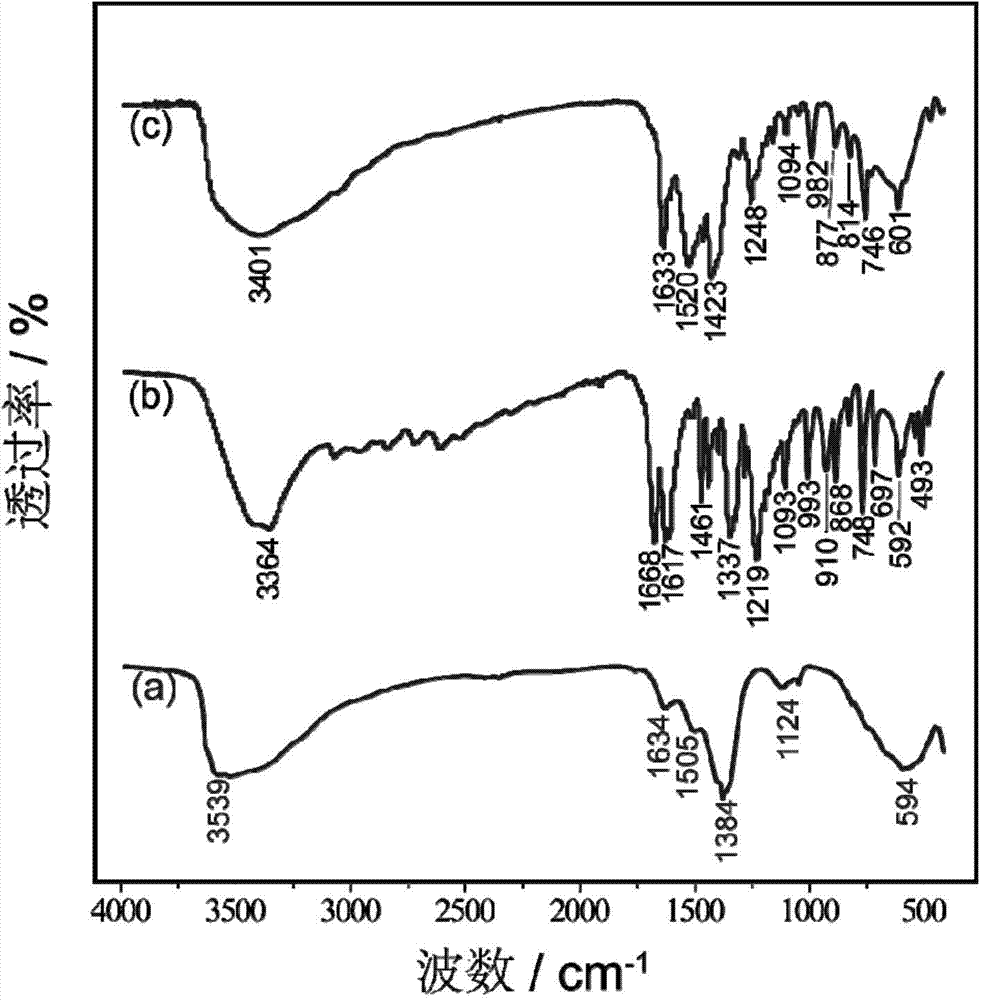
[0052] 0.446g (1mmol) Eu(NO3) 3 ·6H 2 O, 0.846g (10mmol) NaNO 3 , 0.07g (0.5mmol) hexamethylenetetramine is dissolved in 65ml degassed water, obtains aqueous solution, this aqueous solution is transferred in the reactor and feeds N into this aqueous solution 2 3min, then hydrothermal reaction at 70°C for 18 hours. After the reaction, the obtained product was suction filtered, washed with deionized water and then suction filtered twice, and then vacuum-dried at 40° C. for 24 hours to obtain 0.196 g of white powder LEuH. 80% yield based on LEuH.
[0053] Add 0.207g (1.26mmol) of o-coumaric acid and 0.054g (1.26mmol) of NaOH into 10mL of deionized water, and ultrasonically dissolve the o-coumaric acid to obtain a clear aqueous solution of o-coumaric acid sodium salt.
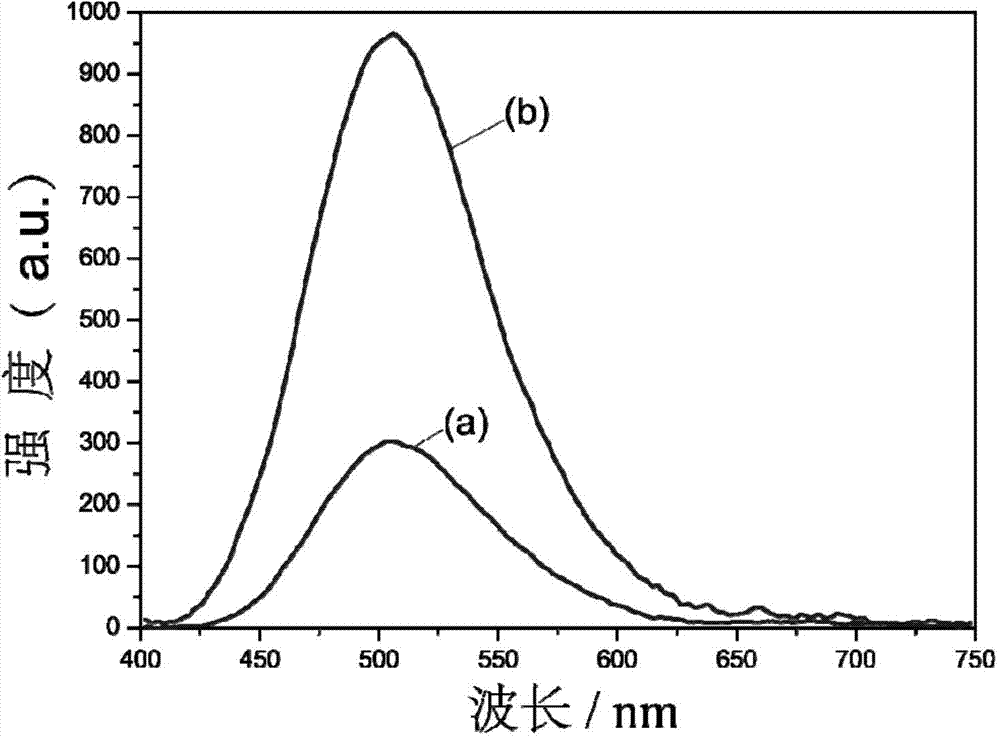
[0054] Disperse 0.10 g (0.42 mmol) of LEuH in 140 ml of deionized water, mix it with the aqueous solution of o-coumaric acid sodium salt prepared above, transfer it to a reaction kettle, and conduct a hydrother...
Embodiment 2
[0056] 0.446g (1mmol) Eu(NO 3 ) 3 ·6H 2 O, 1.10g (13mmol) NaNO 3 , 0.14g (1mmol) hexamethylenetetramine is dissolved in 80ml degassed water, obtains an aqueous solution, this aqueous solution is transferred in the reactor and feeds N in this aqueous solution 2 5min, then 90°C hydrothermal reaction for 14 hours. After the reaction, the obtained product was suction filtered, washed with deionized water and then suction filtered three times, then vacuum dried at 60° C. for 20 hours to obtain 0.240 g of white powder LEuH. Based on LEuH, the yield was 98%.
[0057] Add 0.207g (1.26mmol) of o-coumaric acid and 0.081g (1.9mmol) of NaOH into 15mL of deionized water, and ultrasonically dissolve the o-coumaric acid to obtain a clear aqueous solution of o-coumaric acid sodium salt.
[0058] Disperse 0.05g (0.21mmol) of LEuH in 100ml of deionized water and mix it with the aqueous solution of o-coumaric acid sodium salt prepared above, and transfer it to a reaction kettle for hydrothe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com