Preparation method of porous calcite fluorescent material used for preparing fluorescent sensor
A fluorescent sensor and fluorescent material technology, applied in the direction of luminescent materials, fluorescence/phosphorescence, chemical instruments and methods, etc., can solve the problems of complex instruments, the influence of europium ion light absorption and emission performance, and the sensor is not suitable for actual testing, etc., to achieve increased area, weaken concentration quenching effect, reduce the effect of concentration quenching
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0038] Example 1-1, a method for preparing a macroporous mayenite bulk fluorescent material, the following steps are performed in sequence:
[0039] 1) At room temperature, dissolve 0.04 g of the phase separation inducer polyethylene oxide (PEO, with a molecular weight of 100,000) in a mixed solvent composed of 2.0 ml of water and 2.0 ml of ethanol, stir magnetically, and then add the 1.000g (0.0026mol) aluminum nitrate nonahydrate [Al(NO 3 ) 3 9H 2 O], 0.800g (0.0034mol) calcium nitrate tetrahydrate [Ca(NO 3 ) 2 4H 2 O], 0.2g (0.00055mol) of europium chloride hexahydrate (EuCl 3 ·6H 2 0), and after adding 0.4ml of drying control agent (formamide) and 0.4ml of complexing agent (ethylene glycol) and stirring evenly for 60min, add 2.0ml of gel accelerator (PO) and in an ultrasonic instrument (parameter is frequency 53KHz, Output power 100%, water temperature 20 ℃) in ultrasonic for 60 seconds;
[0040] 2), put the uniform and transparent sol liquid obtained in step 1) ...
Embodiment 1-2
[0048] The phase separation inducer polyethylene oxide in step 1) of Example 1-1 was changed from 0.04 g to 0.12 g; the rest were the same as in Example 1-1.
[0049] Observation by scanning electron microscope at low and high magnifications shows that the obtained mayenite still has a coherent macropore structure, but with the increase of the amount of PEO, the diameter of the macropores decreases as ( figure 2 , about 0.7 μm), the porosity is 74%. This is due to the further progress of PEO phase separation. After the fluorescence test, its luminous intensity decreased slightly (such as Figure 4 ).
Embodiment 1-3
[0051] The phase separation inducer polyethylene oxide in step 1) of Example 1-1 was changed from 0.04 g to 0.24 g; the rest were the same as in Example 1-1.
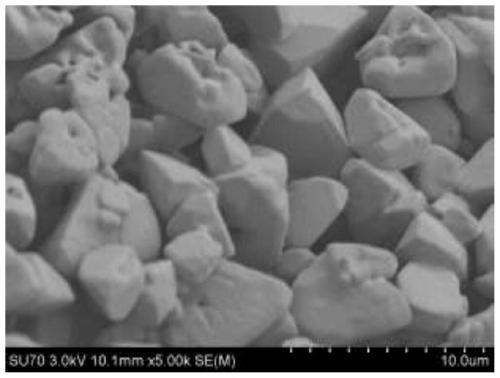
[0052] The low-power and high-power observations of the scanning electron microscope show that the microscopic morphology of the mayenite block has changed to the accumulation of mayenite particles, that is, it does not present a coherent macropore structure such as ( image 3 ). This is because as the amount of PEO increases, the phase separation effect of PEO is further enhanced, and excessive phase separation occurs. After fluorescence test, its luminous intensity further decreased (such as Figure 4 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com