Benzophenothiazine derivative and preparation method thereof
A technology of benzophenothiazine and derivatives, which is applied in the field of organic photoelectric functional materials, can solve the problems of resource scarcity, limited development and application, and high price, and achieve the goal of broadening the spectral response range, improving charge transfer, and low production cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
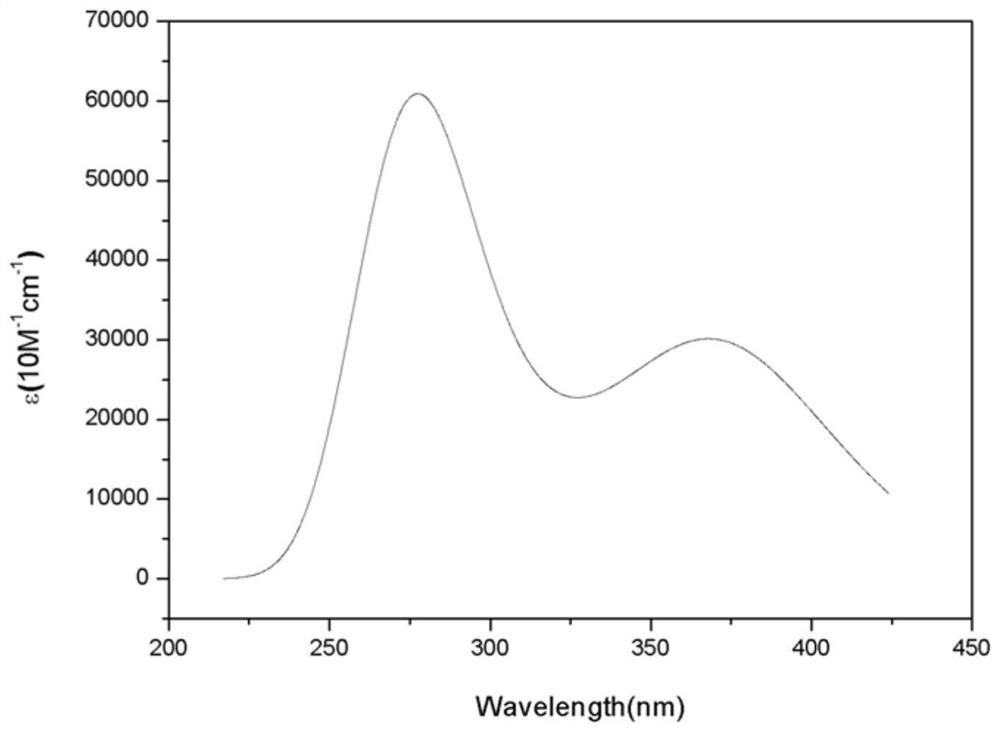
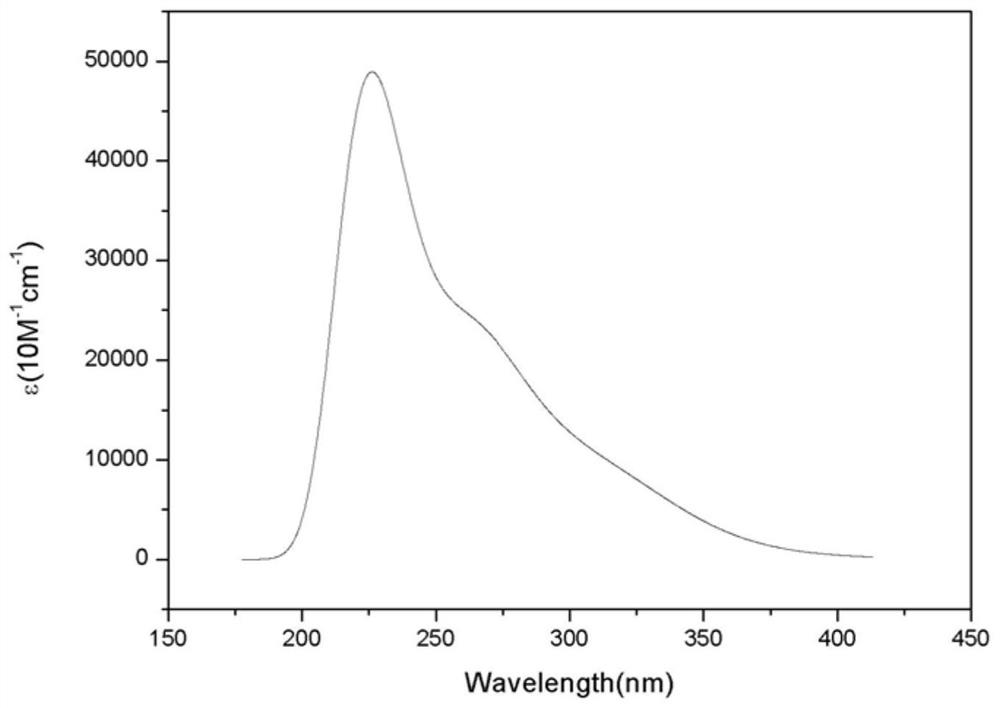
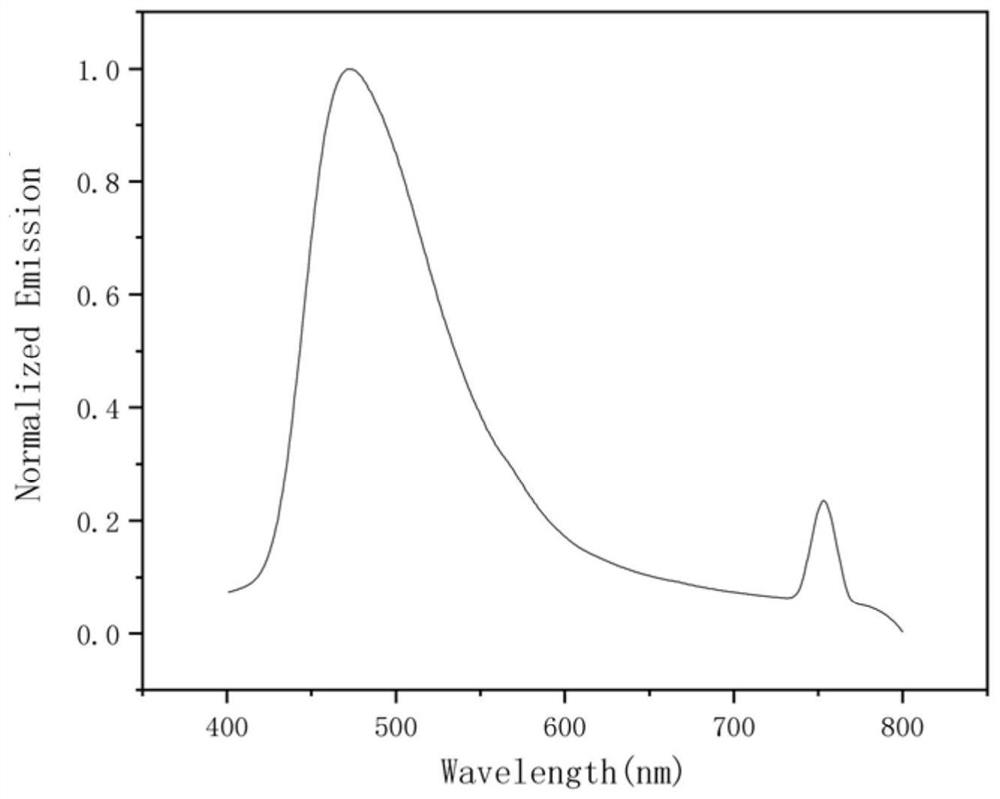
Image
Examples
Embodiment 1
[0039]
[0040] The synthetic route is as follows:
[0041]
[0042] A mixed solution of 2-aminobenzenethiol (0.75 g, 6 mmol) and 1-tetralone (0.584 g, 4 mmol) in DMSO (16.0 mL) was stirred at 110° C. in air for 24 hours. Upon completion, the reaction mixture was diluted with ethyl acetate (64.0 mL) and filtered. The volatiles were removed in vacuo to give crude product. Further separation and purification by silica gel column chromatography (EtOAc / petroleum ether) gave benzophenothiazine 1. Yield: 87%. 1 H NMR (400MHz, Chloroform-d) δ9.58(s,2H),7.89–7.80(m,2H),7.76–7.69(m,2H),7.64(dd,J=7.4,1.5Hz,2H), 7.53–7.42(m,4H),7.29(dd,J=7.4,0.5Hz,2H),7.19–7.15(m,1H),7.15–7.01(m,7H).
[0043]
[0044] In a round bottom flask was added benzophenothiazine 1 (1.246g, 5mmol), 4-bromobenzonitrile (1.092g, 6mmol), sodium tert-butoxide (2.76g, 15mmol), tris(dibenzylideneacetone) Dipalladium (92mg, 0.1mmol), tri-tert-butylphosphine tetrafluoroborate (0.146g, 0.5mmol), toluene 25mL....
Embodiment 2
[0046]
[0047] The synthetic route is as follows:
[0048]
[0049]A mixed solution of 2-aminobenzenethiol (0.75 g, 6 mmol) and 1-tetralone (0.584 g, 4 mmol) in DMSO (16.0 mL) was stirred at 110° C. in air for 24 hours. Upon completion, the reaction mixture was diluted with ethyl acetate (64.0 mL) and filtered. The volatiles were removed in vacuo to give crude product. Further separation and purification by silica gel column chromatography (EtOAc / petroleum ether) gave benzophenothiazine 1. Yield: 87%. 1 H NMR (400MHz, Chloroform-d) δ9.58(s,2H),7.89–7.80(m,2H),7.76–7.69(m,2H),7.64(dd,J=7.4,1.5Hz,2H), 7.53–7.42(m,4H),7.29(dd,J=7.4,0.5Hz,2H),7.19–7.15(m,1H),7.15–7.01(m,7H).
[0050]
[0051] In a round bottom flask was added benzophenothiazine 1 (1.246g, 5mmol), (4-bromophenyl)[bis(2,4,6-trimethylphenyl)]borane (2.424g, 6mmol) , sodium tert-butoxide (2.76g, 15mmol), tris(dibenzylideneacetone) dipalladium (92mg, 0.1mmol), tri-tert-butylphosphine tetrafluoroborate (0...
Embodiment 3
[0053]
[0054] The synthetic route is as follows:
[0055]
[0056] A mixed solution of 2-aminobenzenethiol (0.75 g, 6 mmol) and 1-tetralone (0.584 g, 4 mmol) in DMSO (16.0 mL) was stirred at 110° C. in air for 24 hours. Upon completion, the reaction mixture was diluted with ethyl acetate (64.0 mL) and filtered. The volatiles were removed in vacuo to give crude product. Further separation and purification by silica gel column chromatography (EtOAc / petroleum ether) gave benzophenothiazine 1. Yield: 87%. 1 H NMR (400MHz, Chloroform-d) δ9.58(s,2H),7.89–7.80(m,2H),7.76–7.69(m,2H),7.64(dd,J=7.4,1.5Hz,2H), 7.53–7.42(m,4H),7.29(dd,J=7.4,0.5Hz,2H),7.19–7.15(m,1H),7.15–7.01(m,7H).
[0057]
[0058] Add benzophenothiazine 1 (1.246g, 5mmol), 5-bromo-1,3-benzenedinitrile (1.246g, 6mmol), sodium tert-butoxide (2.76g, 15mmol), three ( Dibenzylideneacetone) dipalladium (92mg, 0.1mmol), tri-tert-butylphosphine tetrafluoroborate (0.146g, 0.5mmol), toluene 25mL. Under the protec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com