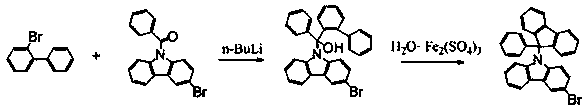
A kind of synthetic method of 3-bromo-9-(9-phenylfluoren-9-yl)carbazole
A synthetic method, benzoyl carbazole technology, applied in the field of organic synthesis, can solve problems such as high hole mobility, achieve high luminous efficiency, vitrification problem improvement, and high thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Under the protection of argon, 5.83 g (25 mmol) of 2-bromobiphenyl and 50 mL of methyl tert-butyl ether were successively added to a 250 mL three-necked flask, and the temperature was lowered to -30°C, and n-butyllithium in n-hexane was added dropwise Solution (2.5 mol / L) 12 mL (30 mmol), keep stirring at -30 ℃ for 0.5 h after the dropwise addition, add dropwise 8.76 g (25 mmol) of 3-bromo-9-benzoylcarbazole and 20 mLTHF to prepare After the reaction is over, add 30 mL of dilute hydrochloric acid aqueous solution to the reaction solution for hydrolysis, separate the organic phase, dry over anhydrous magnesium sulfate, concentrate the solvent under reduced pressure, add 50 mL of toluene, and then add 0.523 g of ferric sulfate monohydrate, Raise the temperature to 40°C and stir for 72 h. After the reaction, add 30 mL of water to the reaction liquid, separate the organic phase, extract the water layer with 30 mL of toluene, dry over anhydrous magnesium sulfate, and recover ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com