Anti-HIV-1 drugs and preparation and application thereof
An HIV-1, drug technology, applied in antiviral agents, pharmaceutical formulations, chemical instruments and methods, etc., can solve the problems of low efficiency, adverse reactions, drug resistance, huge medical costs, drug side effects, etc., and achieve the effect of avoiding mutation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Construction model of chimeric vector Rev-Vif-C
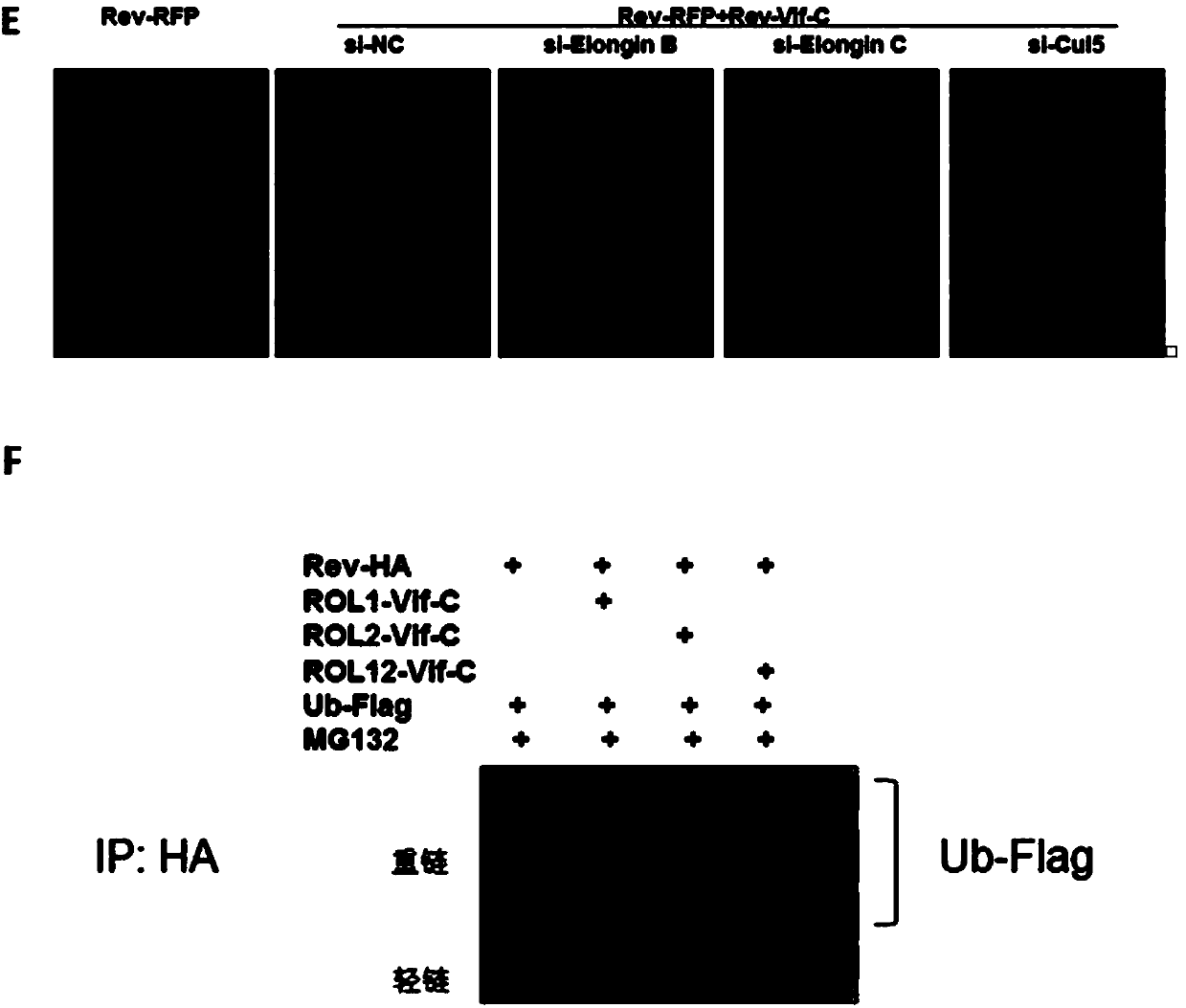
[0031] The two oligomerization binding domains (amino acids 9-26 and amino acids 51-65) on the HIV-1 Rev gene were respectively cloned into the N-terminus of the Vif gene, and replaced with the binding site of APOBEC3G (1- 79 amino acids), and then connected to the pcDNA3.1 vector to form three different chimeric chimeras, which were named ROL1-Vif-C, ROL2-Vif-C, and ROL12-Vif-C. Among them, ROL1 represents the oligomerization binding domain of the N-terminal part of Rev, that is, amino acids 9-26; ROL2 represents the oligomerization binding domain of the C-terminal part of Rev, that is, amino acids 51-65; ROL12 represents the tandem Two oligomerization binding domains at the N-terminal and C-terminal of Rev, namely amino acids 9-26 and amino acids 51-65.
[0032] Specific steps are as follows:
[0033] (1) There are two oligomerization domains in the protein structure of Rev, and these two oligomerization do...
Embodiment 2
[0036] Example 2 The chimeric vector Rev-Vif-C degrades Rev protein through the ubiquitination pathway
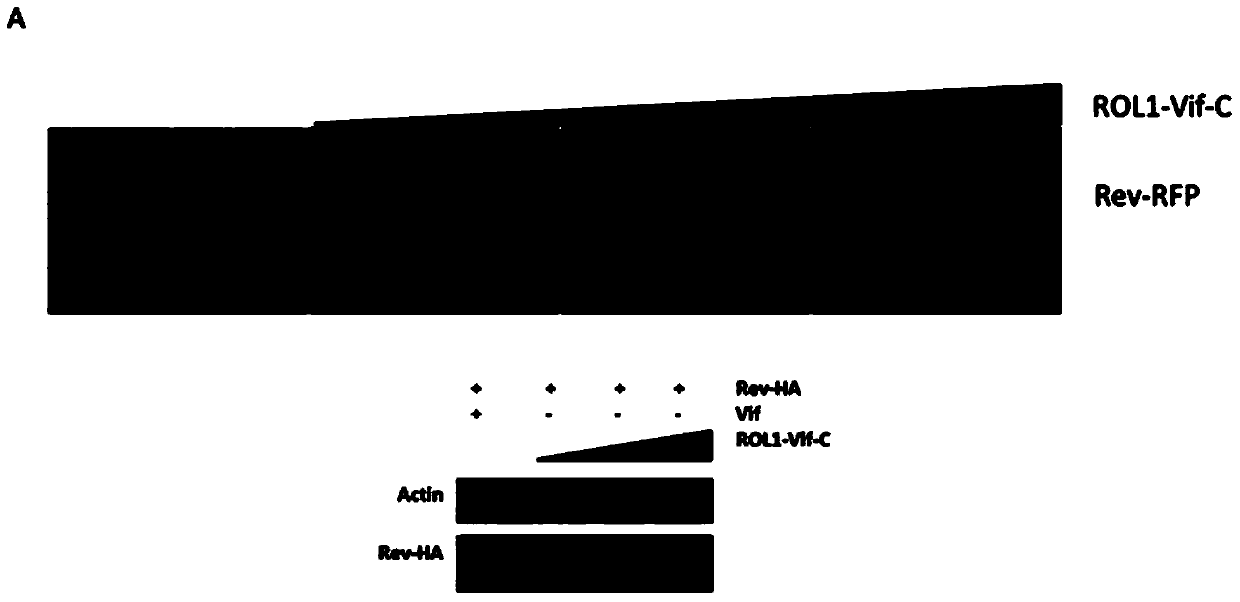
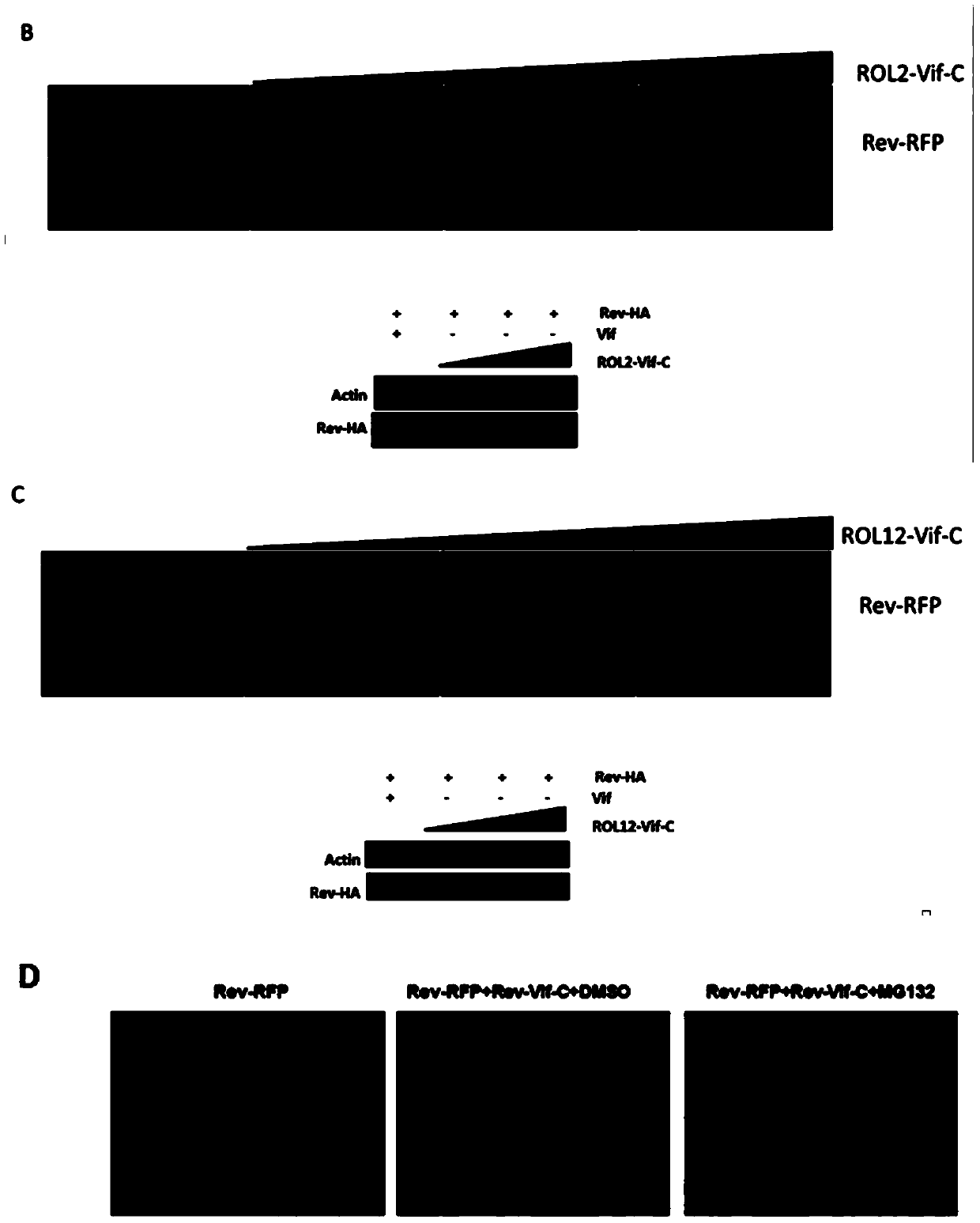
[0037] Fusion expression of HIV-1 Rev gene and RFP and cloned into the vector of pcDNA3.1, so that the expression of Rev can be reflected by observing the expression of RFP; at the same time, the fusion expression of Rev gene and HA tag is convenient for western blot to further Verify the expression of Rev.
[0038](1) In 293t cells in a 24-well plate, co-transfect Rev-RFP and ROL1-Vif-C (or ROL2-Vif-C or ROL12-Vif-C) vectors, the amount of plasmids was 1:0, 1 : 1, 1: 2, 1: 3, observe the expression of RFP 48 hours after transfection, and co-transfect Rev-HA and ROL1-Vif-C vectors in 293t cells in a 6-well plate, and the amount of plasmids is 1 :0, 1:1, 1:2, 1:3, 48 hours after transfection, the cells were harvested and lysed for Western Blot to detect the expression of Rev
[0039] This experiment shows that all three chimeric vectors can inhibit the expression of Rev pr...
Embodiment 3
[0044] Example 3 The chimeric vector Rev-Vif-C inhibits the replication of various wild-type HIV-1 strains by inhibiting the nuclear export function of Rev-RRE
[0045] There are SD and SA cleavage sites on PDM628. When the Rev protein does not exist, the luciferase gene carried on PDM 628 is clipped, resulting in a small amount of luciferase expression; when the two plasmids are co-transfected, Rev and RRE combine to convert luciferase The gene fragments are taken out of the nucleus, avoiding being edited by SD and SA, so that luciferase can be expressed in large quantities. Therefore, when Rev protein expression is inhibited, the Rev-RRE-related nuclear export system will be inhibited, and the expression of luciferase will decrease at this time. Based on this principle, it can be judged whether the function of the Rev protein will be affected.
[0046] (1) In 293t cells in a 96-well plate, 10 ng of pDM628 plasmid and 10 ng of Rev plasmid were co-transfected, and then co-tra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com