Synthetic method of 5-amino-4-carbamyl imidazole ribavirin carbocyclic analog
A technology of carbamoyl imidazole riboside carbon and synthesis method, which is applied in the field of synthesis of 5-amino-4-carbamyl imidazole riboside carbocyclic analogs, can solve the problem of harsh synthesis method conditions, high synthesis reaction cost and environmental problems. Serious pollution and other problems, to achieve the effect of good environmental protection, fewer reaction steps, and high reaction quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0018] The following will clearly and completely describe the technical solutions in the embodiments of the present invention. Obviously, the described embodiments are only some of the embodiments of the present invention, rather than all the embodiments. Based on the embodiments of the present invention, all other embodiments obtained by persons of ordinary skill in the art without making creative efforts belong to the protection scope of the present invention.
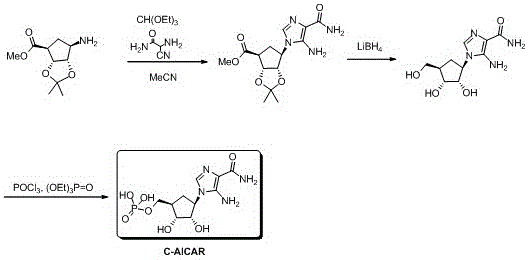
[0019] see figure 1 , provide a kind of synthetic method of 5-amino-4-carbamoyl imidazolidine carbocyclic analogue, comprising the steps of:
[0020] (1) 0.24 g, 1.1 mmol of (3aR,4S,6R,6aS)-methyl-6-amino-2,2-dimethyltetrahydro-3ahydro-cyclopenta[d][1,3] Dioxy-4-carboxylate was dissolved in 5 mL of acetonitrile, and 0.19 g, 1.3 mmol of triethyl orthoformate and 0.13 g, 1.3 mmol of 2-amino-2-cyanoacetamide were added in sequence. The reaction solution was heated to reflux in acetonitrile for 12 hours, cooled, added ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com