Anti-tumor medicament as well as synthetic method and application thereof
A technology of pharmacy and compounds, applied in antitumor drugs, drug combinations, pharmaceutical formulations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
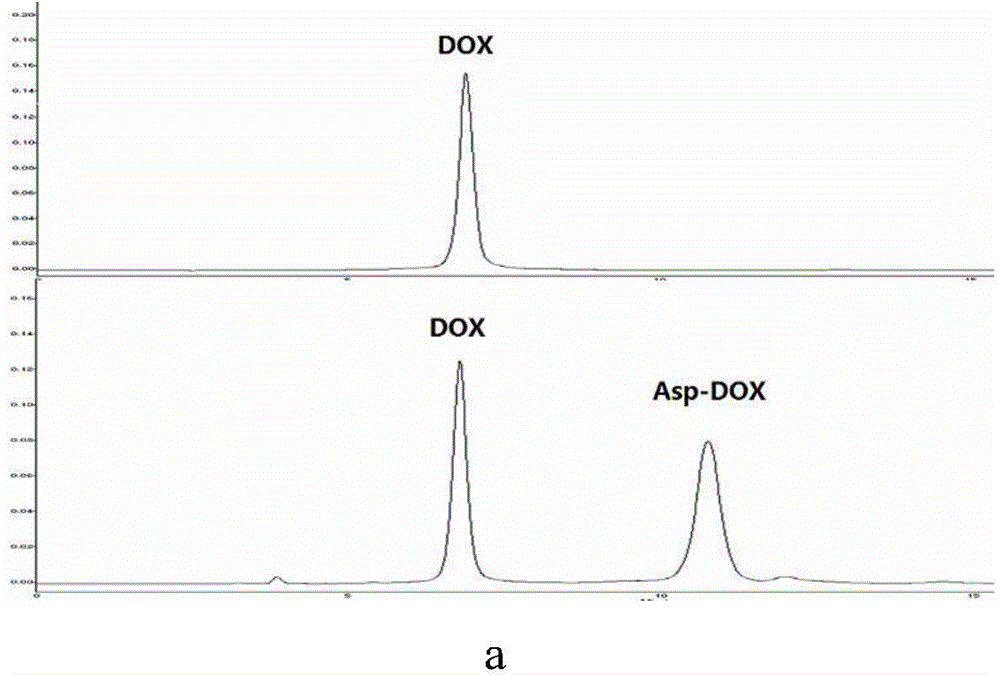
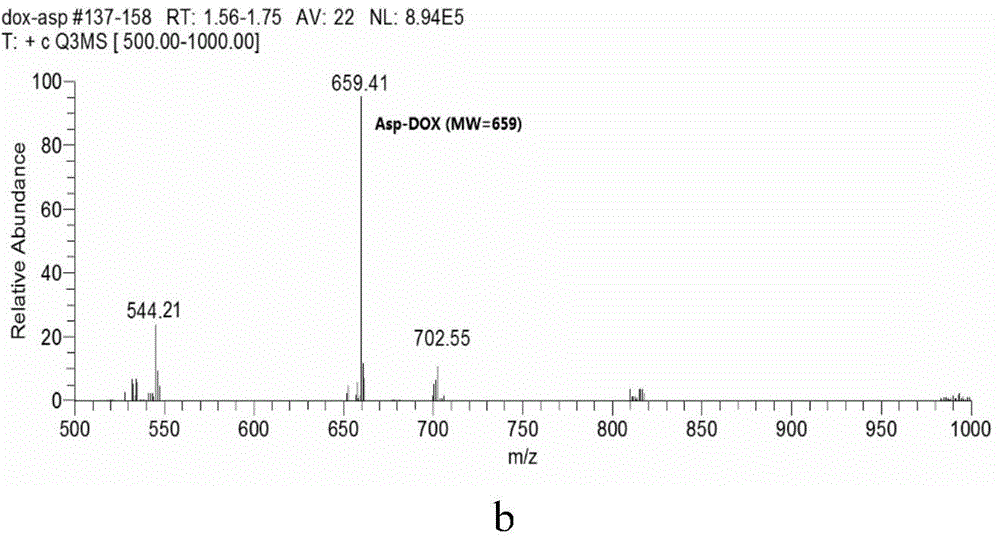
[0047] MS was measured with 4000 QTRAP? LC / MS / MS instrument from AB SCIEX Company, and all were EI sources (70ev) unless otherwise specified; all solvents were redistilled before use, and the anhydrous solvents used were all according to standard The method was obtained by drying; unless otherwise specified, all reactions were carried out at room temperature and followed by TLC. The purification of the product used silica gel (300-400 mesh) column chromatography unless otherwise specified; among them, silica gel (300-400 mesh) Produced by Qingdao Ocean Chemical Factory, GF254 thin-layer silica gel plate is produced by Yantai Jiangyou Silicone Development Co., Ltd.; the prepared product was confirmed by HPLC and MS.
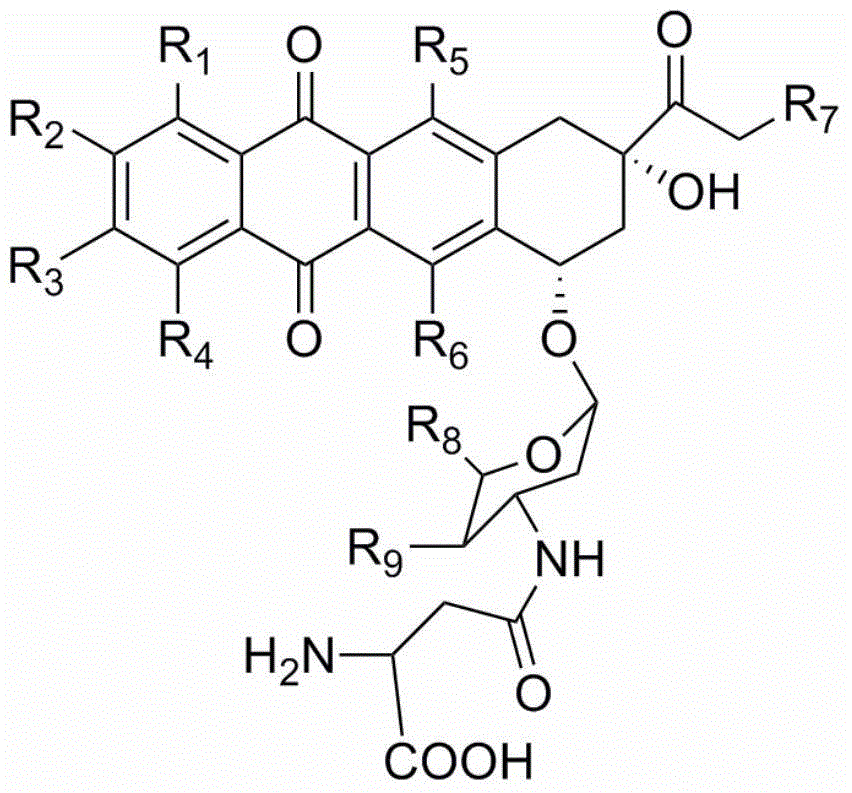
[0048] The preparation of the compound of formula II (doxorubicin-aspartic acid / Doxorubicin-Asp / Asp-DOX), the preparation route is as follows:
[0049]
[0050] The preparation method is prepared by reacting doxorubicin and Fmoc-Asp-OALL with the participation ...
Embodiment 2
[0056] Compound doxorubicin-aspartic acid hydrochloride: Take 1.5 g of the compound purple solid product and dissolve it in 10 mL of absolute ethanol. Cool in an ice-water bath to 5°C, add 11.1% hydrochloric acid ethanol solution dropwise until the pH is 2, and continue to stir for about 1 h in an ice-water bath. Filter and dry in vacuo to obtain a purple solid powder.
Embodiment 3
[0058] Compound doxorubicin-aspartic acid into taurate: Take 2.0 g of the purple solid product of the compound and dissolve it in 10 mL of acetone. After heating to reflux, equimolar taurine was added, and the reaction was stirred for about 1.5 h under reflux. After the reaction was complete, it was left to stand at room temperature for 24 h. Precipitate purple crystals, filter and dry in vacuum to obtain the taurine salt of I.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com