Pharmaceutical composition for treating and/or preventing cancer
A composition and drug technology, applied in the direction of drug combination, medical preparations containing active ingredients, anti-tumor drugs, etc., can solve the problems of normal cell toxicity and side effects of antigens
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0074] Antigens can be prepared by, for example, a method using animal cells (JP 2007-530068) and / or a method using baculovirus (eg, International Publication No. WO98 / 46777, etc.). When the immunogenicity of the antigen is low, it can be combined with a macromolecule with immunogenicity such as albumin for immunization. Antigens can also be administered together with an adjuvant for immunization.
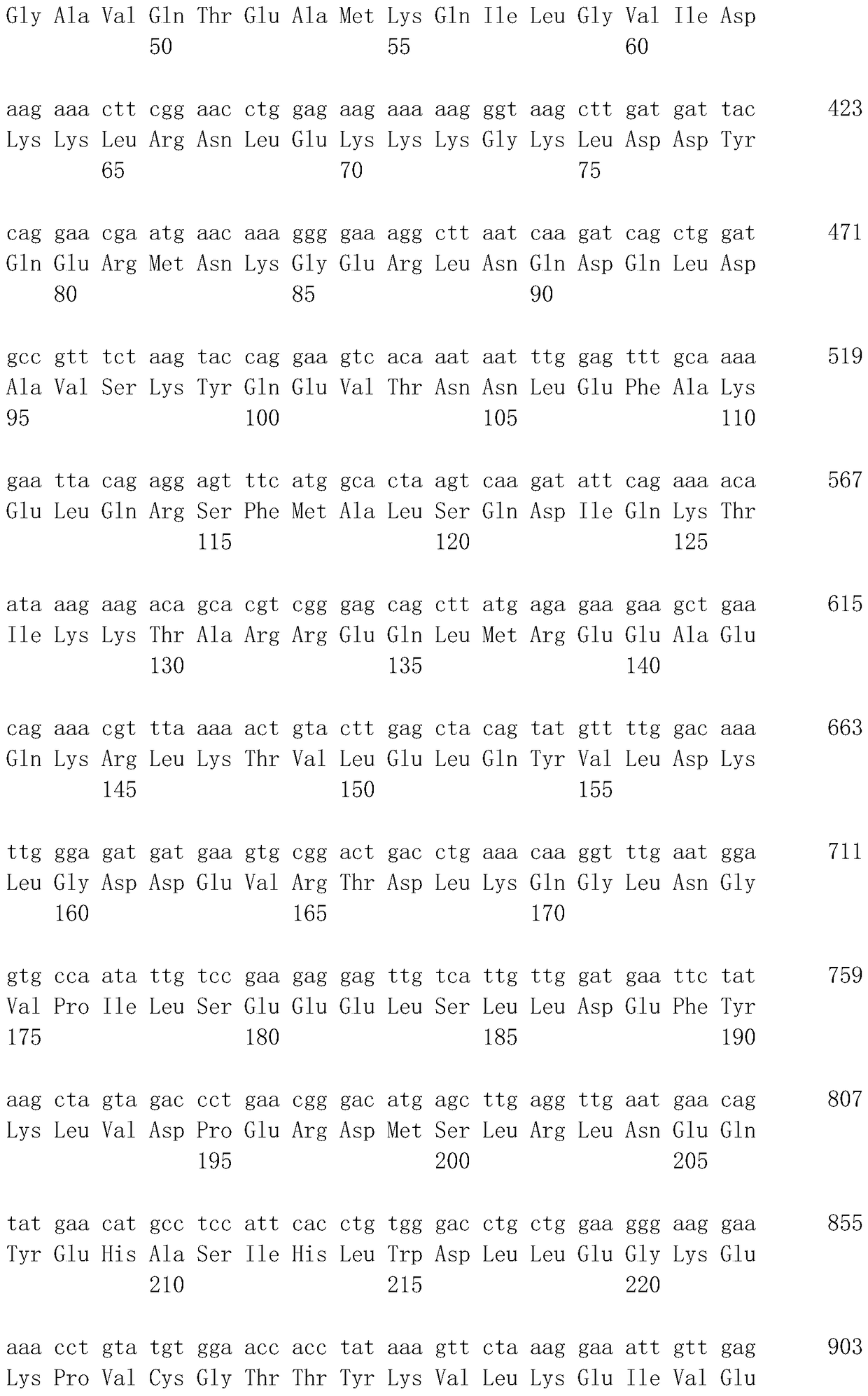
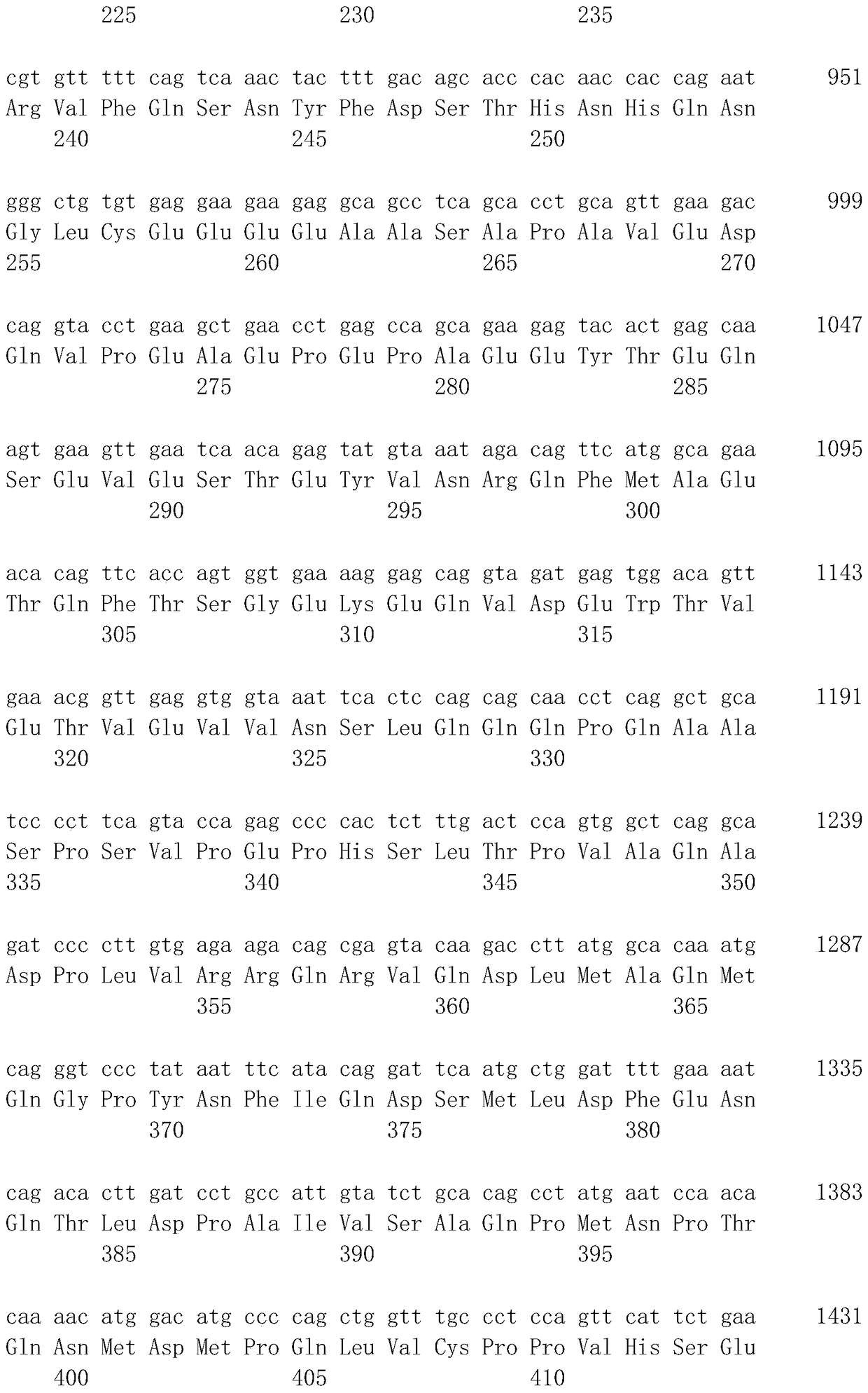
[0075]The antibody of the present invention can also be obtained as a genetically recombinant antibody produced by cloning its antibody gene from a hybridoma, integrating it into an appropriate vector, introducing it into a host, and using genetic recombination technology (see, for example, Carl, A.K. Borrebaeck, James, W. Larrick, THERAPEUTIC MONOCLONAL ANTIBODIES, Published in the United Kingdom by MACMILLAN PUBLISHERS LTD, 1990). Specifically, the cDNA of the antibody variable region (V region) is synthesized from hybridoma mRNA using reverse transcriptase. If DNA encoding the...
Embodiment 1
[0168] CAPRIN-1 expression analysis in each tissue of embodiment 1
[0169] According to Example 1(4) of WO2010 / 016526, the expression of CAPRIN-1 gene in normal tissues and various cell lines of dogs and humans was examined by RT-PCR method. As a result, it was found that, for normal dog tissues, strong expression was found in the testis, and on the other hand, expression was found in dog mammary carcinoma and adenocarcinoma tissues. Furthermore, the expression in human tissues was confirmed at the same time. As a result, similar to the dog CAPRIN-1 gene, only the testis was confirmed to be expressed in normal tissues. For cancer cells, eight human breast cancer cell lines (ZR75-1, MCF7, T47D, SK-BR-3, MDA-MB-157, BT-20, MDA-MB-231V, MRK-nu-1) and 4 pancreatic cancer cell lines (Capan-2, MIAPaCa-2, Panc- 1, BxPc-3) and other cancer cell lines were detected to express. From these results, it was confirmed that CAPRIN-1 was not expressed in normal tissues other than the testi...
Embodiment 2
[0170] Example 2 Production of Mouse Monoclonal Antibody Against CAPRIN-1
[0171] (1) Preparation of mouse monoclonal antibody
[0172] 100 μg of the human CAPRIN-1 protein having the amino acid sequence of SEQ ID NO: 2 prepared in Example 3 of WO2010 / 016526 was mixed with an equal amount of MPL+TDM adjuvant (manufactured by Sigma Corporation), and the resulting mixture was used as a small mouse antigen solution. The antigen solution was intraperitoneally administered to 6-week-old Balb / cc mice (manufactured by Japan SLC), and then administered seven times every week to complete immunization. Each spleen extracted 3 days after the last immunization was sandwiched between two sterilized glass slides and ground, washed with PBS (-) (manufactured by Nissui Co., Ltd.), centrifuged at 1500 rpm for 10 minutes, and The operation of removing the supernatant was repeated three times to obtain spleen cells. The obtained spleen cells were mixed with mouse myeloma cells SP2 / 0 (purchas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com