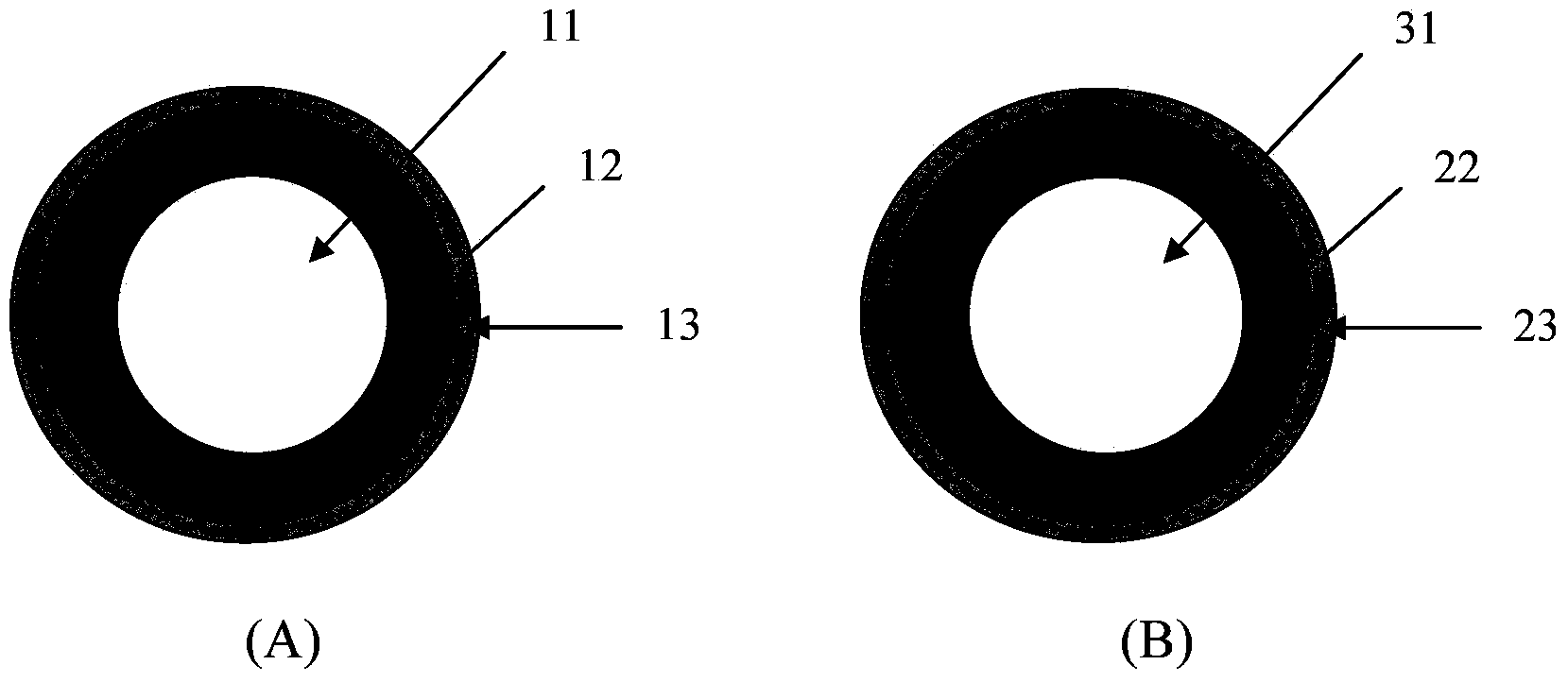Dabigatran etexilate medicine composition and preparation method thereof
A technology of dabigatran etexilate and composition, applied in the field of dabigatran etexilate pharmaceutical composition and its preparation, capable of solving problems such as poor stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-11
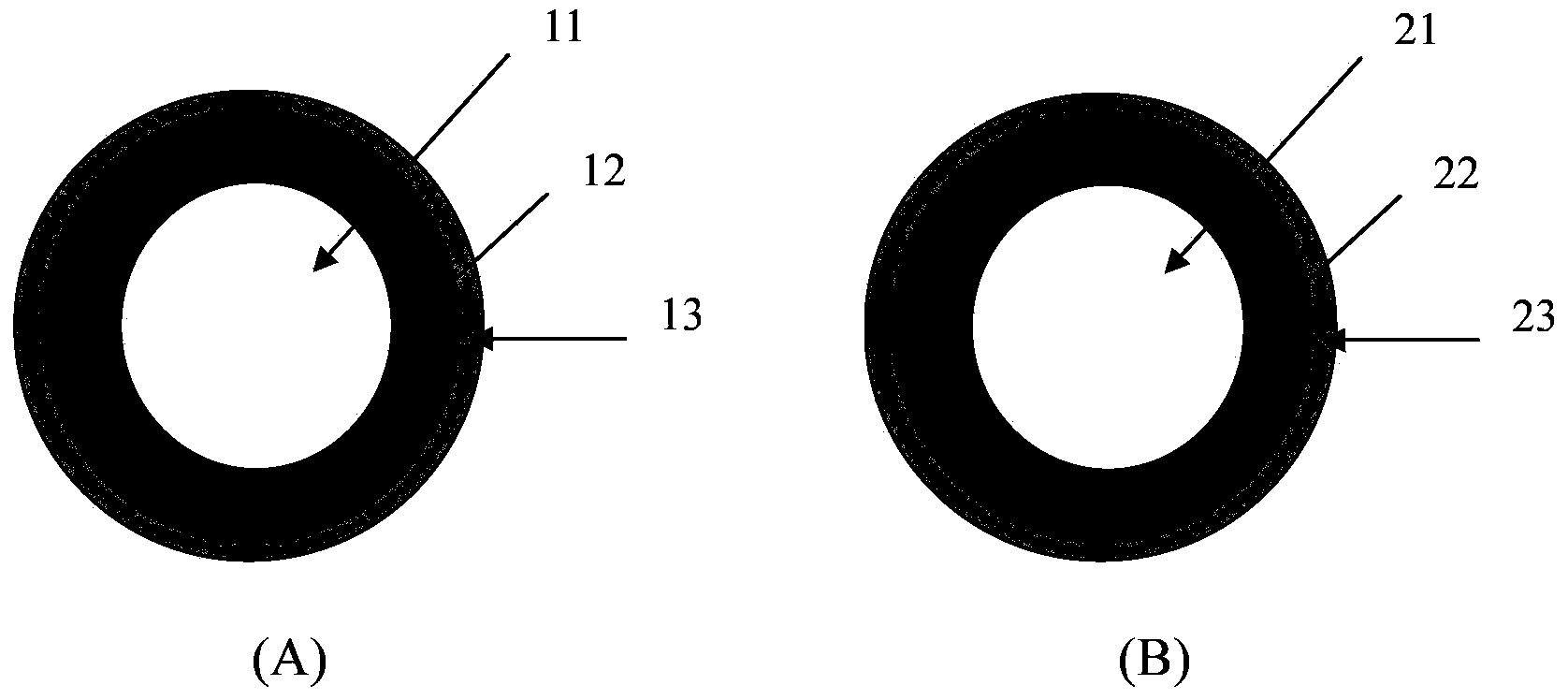
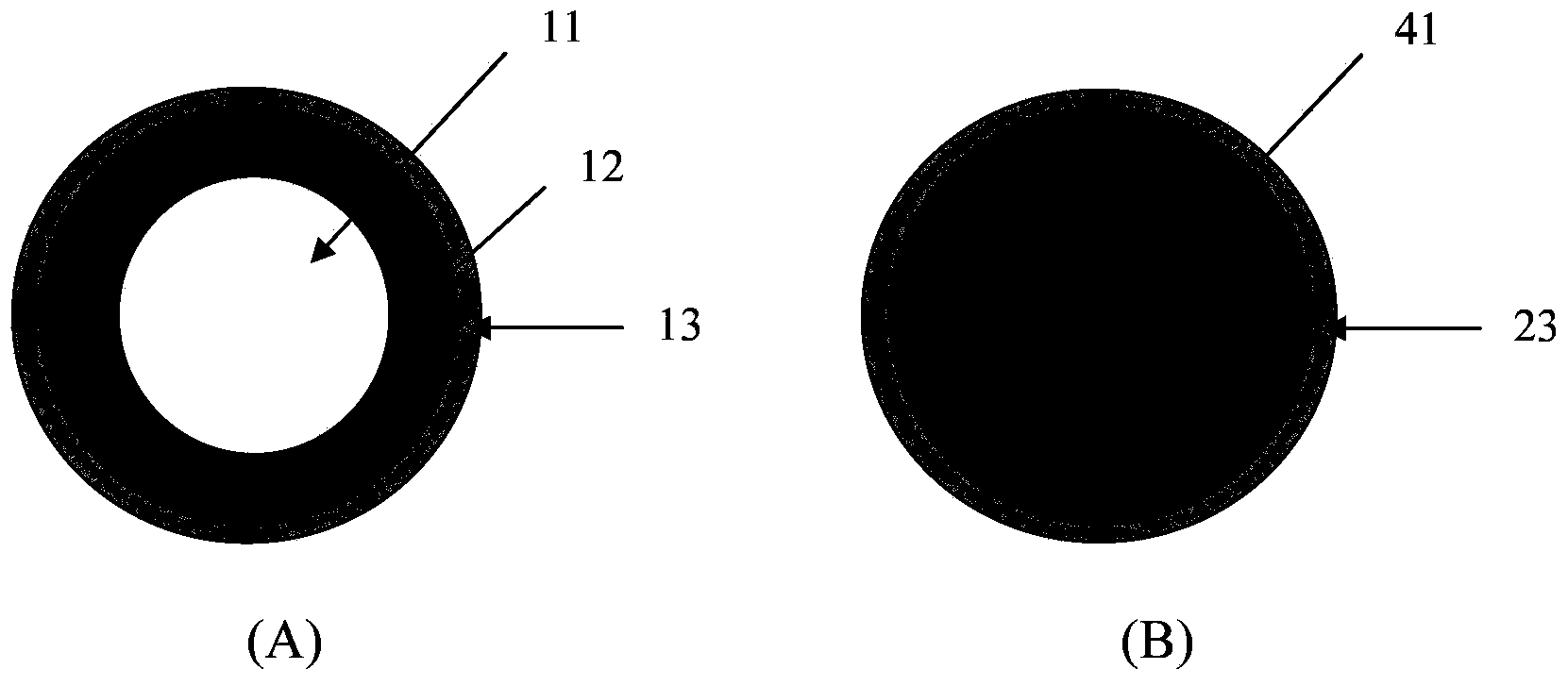
[0141] Will prepare pill 1 (as figure 1 , figure 2 and image 3 Shown in A) component, proportioning are listed in table 1, table 2 and table 3, and wherein wetting agent Virahol just uses in the preparation process, will be removed in final ball 1.
[0142]
[0143]
[0144] The preparation method of pill I in the above-mentioned embodiment 1-10 is as follows:
[0145] (a), dissolving polyvinylpyrrolidone k30 in isopropanol, as a binder solution, its binder concentration is about 2%;
[0146] (b), dabigatran etexilate mesylate or dabigatran etexilate butanedisulfonate particle size distribution is controlled at D90 less than 20 microns, mixed with talcum powder;
[0147] (c), suspending Opadry 200 in isopropanol as the isolation material suspension, the solid content of the suspension is about 8%;
[0148] (d), adopt the centrifugal pill making device, make its turntable be the state of horizontal rotation, its rotating speed is 200 revolutions / min, put blank ball ...
Embodiment 12-25
[0161] Components and proportions for preparing pill II are listed in Table 4, Table 5, Table 6 and Table 7, wherein the wetting agent ethanol is only used in the preparation process and will be removed in the final pill II.
[0162]
[0163]
[0164]
[0165] Pill II in the above-mentioned embodiment 12-24 (as figure 1 Shown in B) the preparation method is as follows:
[0166] (a), dissolving polyvinylpyrrolidone k30 in 50% ethanol as a binder solution, the binder concentration is about 2%;
[0167] (b), the particle size distribution of tartaric acid or maleic acid is controlled at D90 and is less than 150 microns, mixes evenly with talcum powder;
[0168] (c), suspending Opadry 200 in 50% ethanol aqueous solution as the isolation material suspension, the solid content of the suspension is about 8%;
[0169] (d), adopt the centrifugal pill making device, make its turntable be the state of horizontal rotation, its rotating speed is 250 revolutions per minute, put t...
Embodiment 26-37
[0191] Containing dabigatran etexilate in the following pharmaceutical composition is 75mg, and the composition of pill I and pill II, proportioning are listed in table 8, table 9 and table 10 in this composition:
[0192]
[0193]
[0194] The preparation method of composition in above-mentioned embodiment 26-37 is as follows:
[0195] According to the content of dabigatran etexilate in pill I and the content of organic acid in pill II, and according to the different mass ratios of dabigatran etexilate and organic acid, pills I and pill II are filled in No. 1 capsule by 2 filling methods Among them, multiple specifications of dabigatran etexilate capsules (such as 75mg, 110mg, and 150mg, different specifications are only different in loading capacity) can be obtained.
[0196]
[0197] The preparation method of composition among the above-mentioned embodiment 38 is as follows:
[0198] According to the content of dabigatran etexilate in pill I, according to the mass...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com