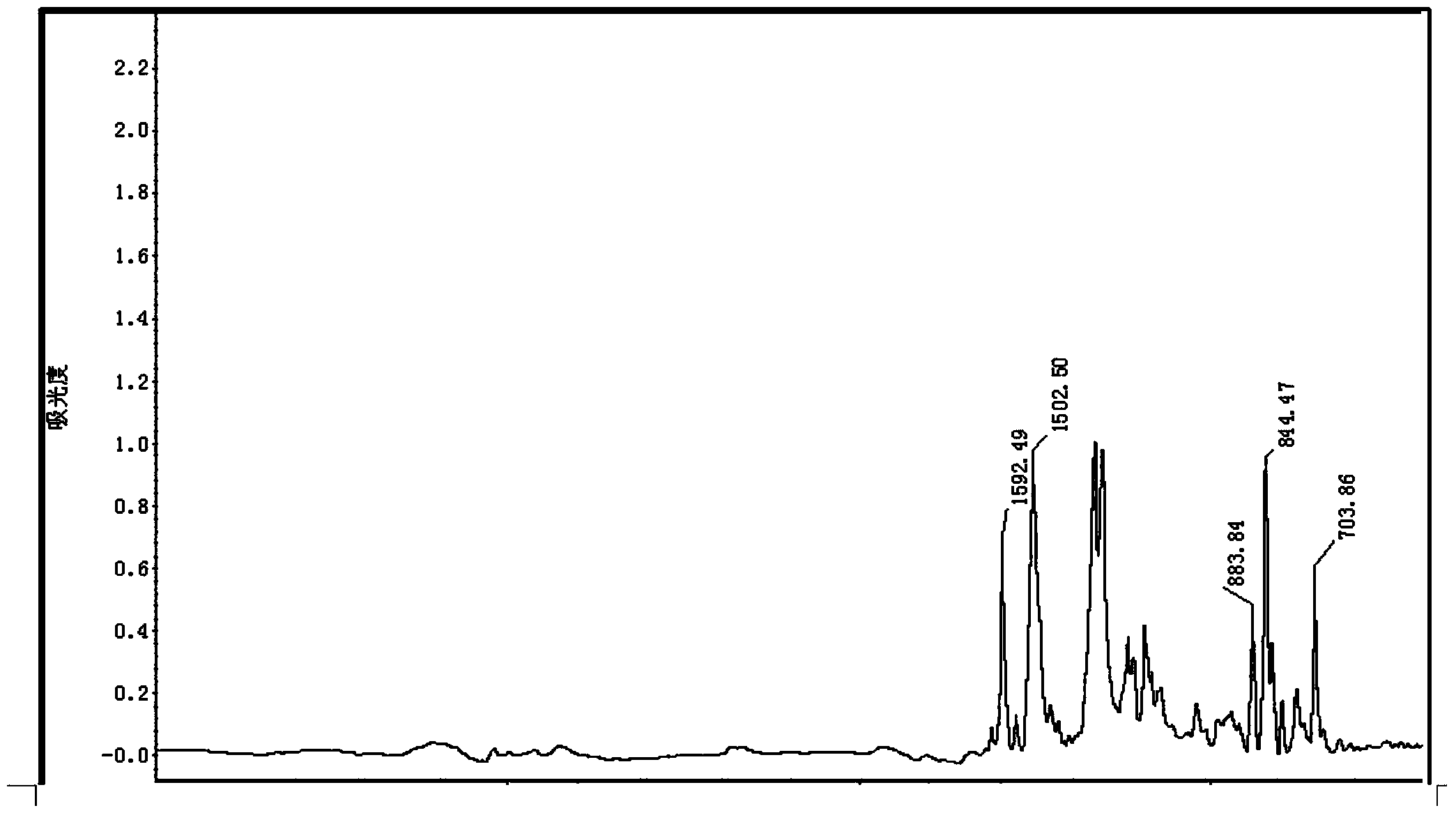
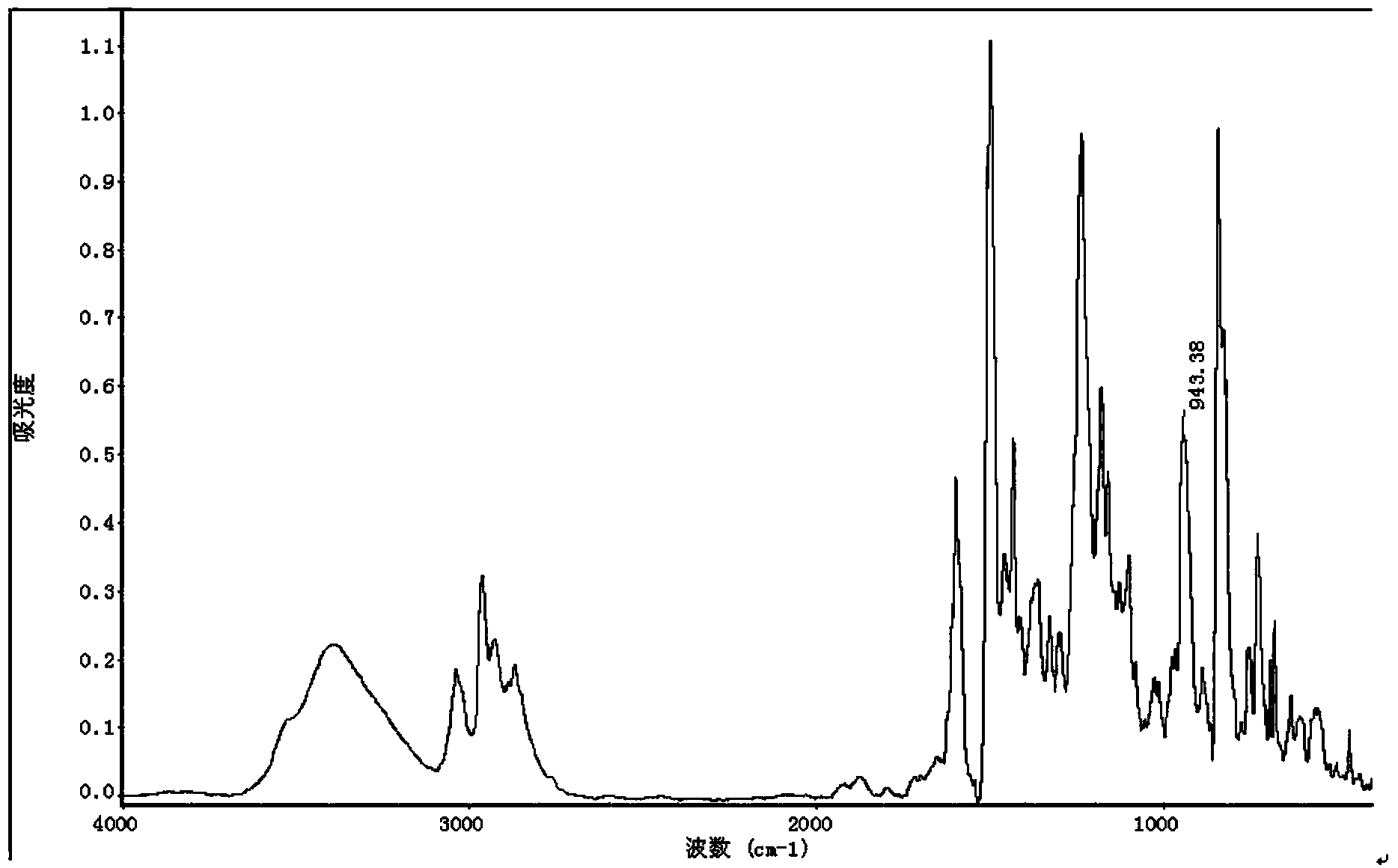
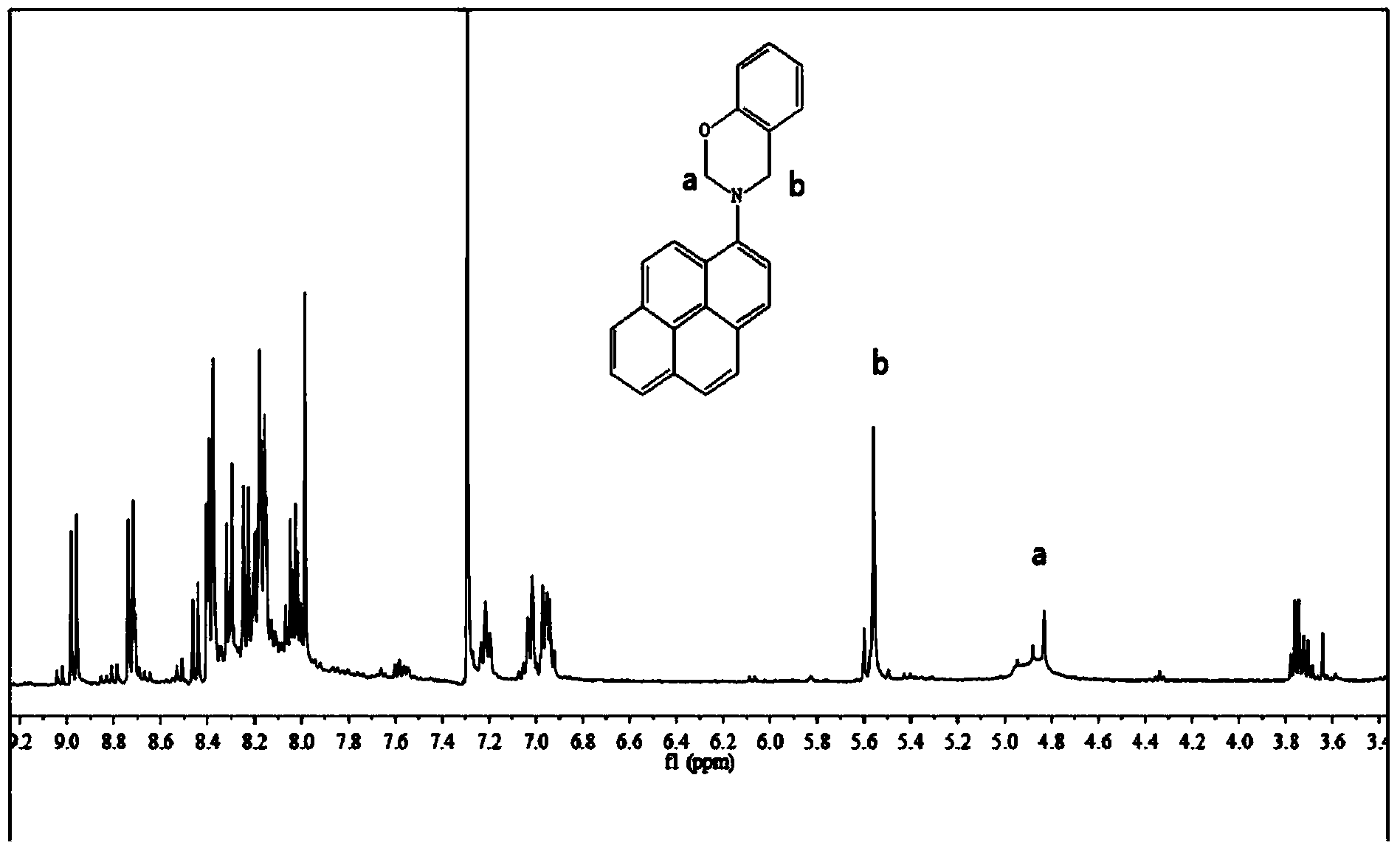
Aminopyrene type benzoxazine compound and preparation method thereof
A technology for benzoxazine and compounds, which is applied in the field of benzoxazine compounds containing pyrene substituents and its preparation, can solve problems affecting the full play of properties, carbon nanotube structure damage, poor compatibility, etc., and achieve a wide range of Application prospects, the effect of good interface compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Adopt solution method to prepare 3,4-dihydro-3-pyrenyl-1,3-benzoxazine, i.e. pyreneamine-phenol type benzoxazine, its structural formula is as follows:
[0057]
[0058] Add pyreneamine (2.17g, 10.0mmol) and phenol (0.94g, 10.0mmol) into a three-necked flask at room temperature, stir to dissolve in about 100ml of toluene. Add paraformaldehyde (0.6g, 20.0mmol) into the three-necked flask in batches, continue to stir to make the mixture uniform, and then gradually raise the temperature to 95°C for reflux. The reaction stopped after 3 hours, and was left to cool to obtain a reddish-brown solution. After washing with 1mol / L NaOH and deionized water, toluene was removed by rotary evaporation, and then vacuum-dried for 24 hours, the obtained reddish-brown crystal product was pyreneamine-phenol-type benzoxazine with a yield of 75.8%.
Embodiment 2
[0060] Prepare 2,2-bis(3,4-dihydro-3-pyrenyl-1,3-benzoxazine) propane by solution method, i.e. pyreneamine-bisphenol A type benzoxazine, its simplified structure as follows:
[0061]
[0062]Add pyreneamine (4.34g, 20.0mmol) and bisphenol A (2.28g, 10.0mmol) into a three-necked flask at room temperature, stir to dissolve in about 96ml of toluene. Add paraformaldehyde (1.2g, 40.0mmol) into the three-necked flask in batches, continue stirring to make the mixture uniform, and then gradually raise the temperature to 95°C for reflux. The reaction stopped after 3 hours, and was left to cool to obtain a reddish-brown solution. After washing with 1mol / L NaOH and deionized water, the toluene was removed by rotary evaporation, and then vacuum-dried for 24 hours. The obtained reddish-brown crystal product was pyrenamine-bisphenol A benzoxazine with a yield of 75.5%.
Embodiment 3
[0063] Embodiment 3 adopts solution method to prepare i.e. pyreneamine-p-cresol type benzoxazine, and its structural formula is as follows:
[0064]
[0065] Add pyreneamine (2.17g, 10.0mmol) and p-cresol (1.08g, 10.0mmol) into a three-necked flask at room temperature, and stir to dissolve in 100ml of toluene. Add paraformaldehyde (0.6g, 20.0mmol) into the three-necked flask in batches, continue to stir to make the mixture uniform, and then gradually raise the temperature to 95°C for reflux. The reaction stopped after 3 hours, and was left to cool to obtain a reddish-brown solution. After washing with 1mol / L NaOH and deionized water, the toluene was removed by rotary evaporation, and then vacuum-dried for 24 hours to obtain a reddish-brown crystal product which was pyreneamine-p-cresol benzoxazine with a yield of 65.5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com