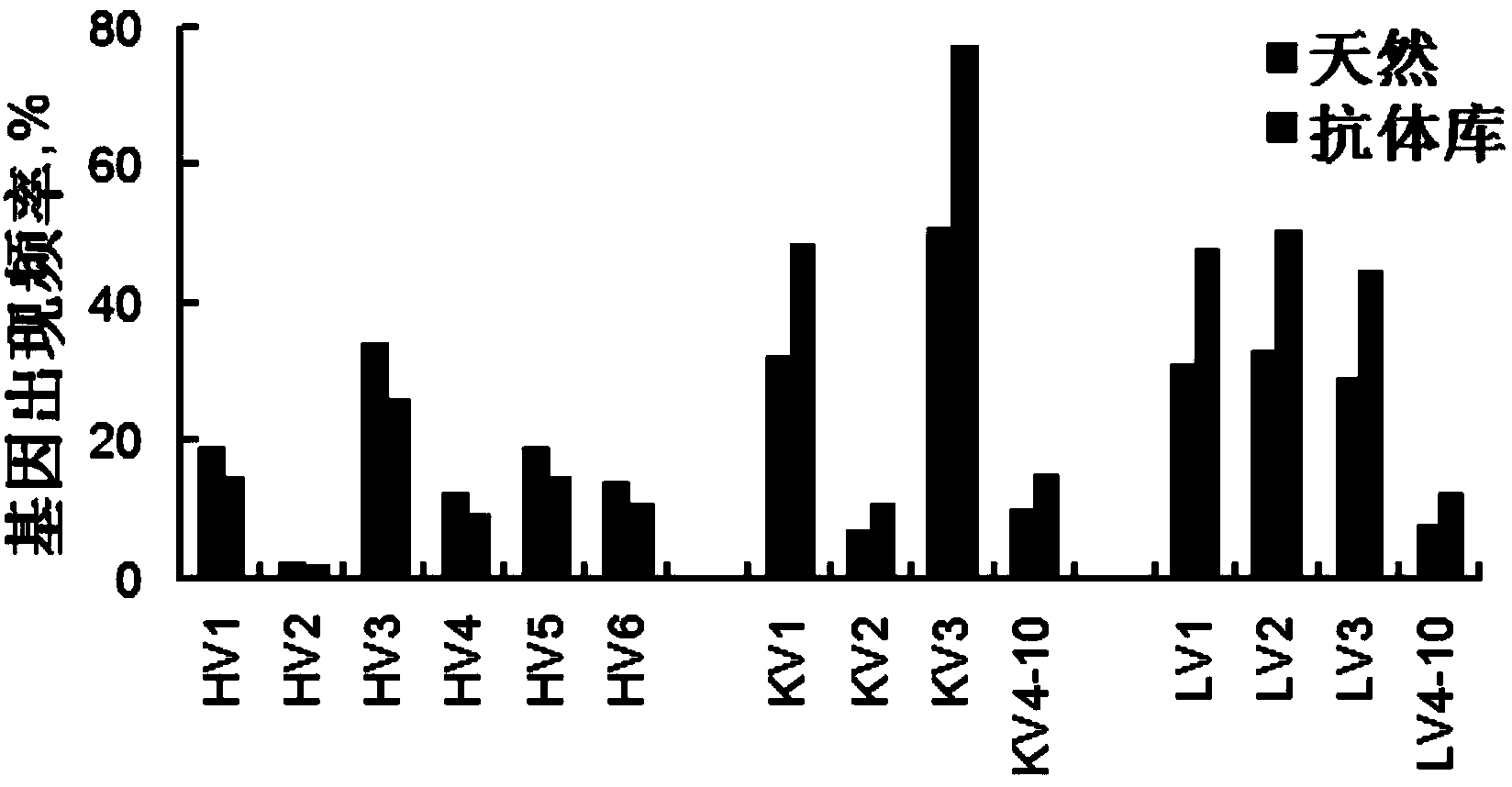Fully-humanized anti-human interleukin 17A single-chain antibody
A technology of interleukin and antibody, which is applied in the field of medicine, can solve problems such as inability to effectively cause CDC
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0089] Preparation Example 1 Construction of Large Capacity Natural Phage Antibody Library
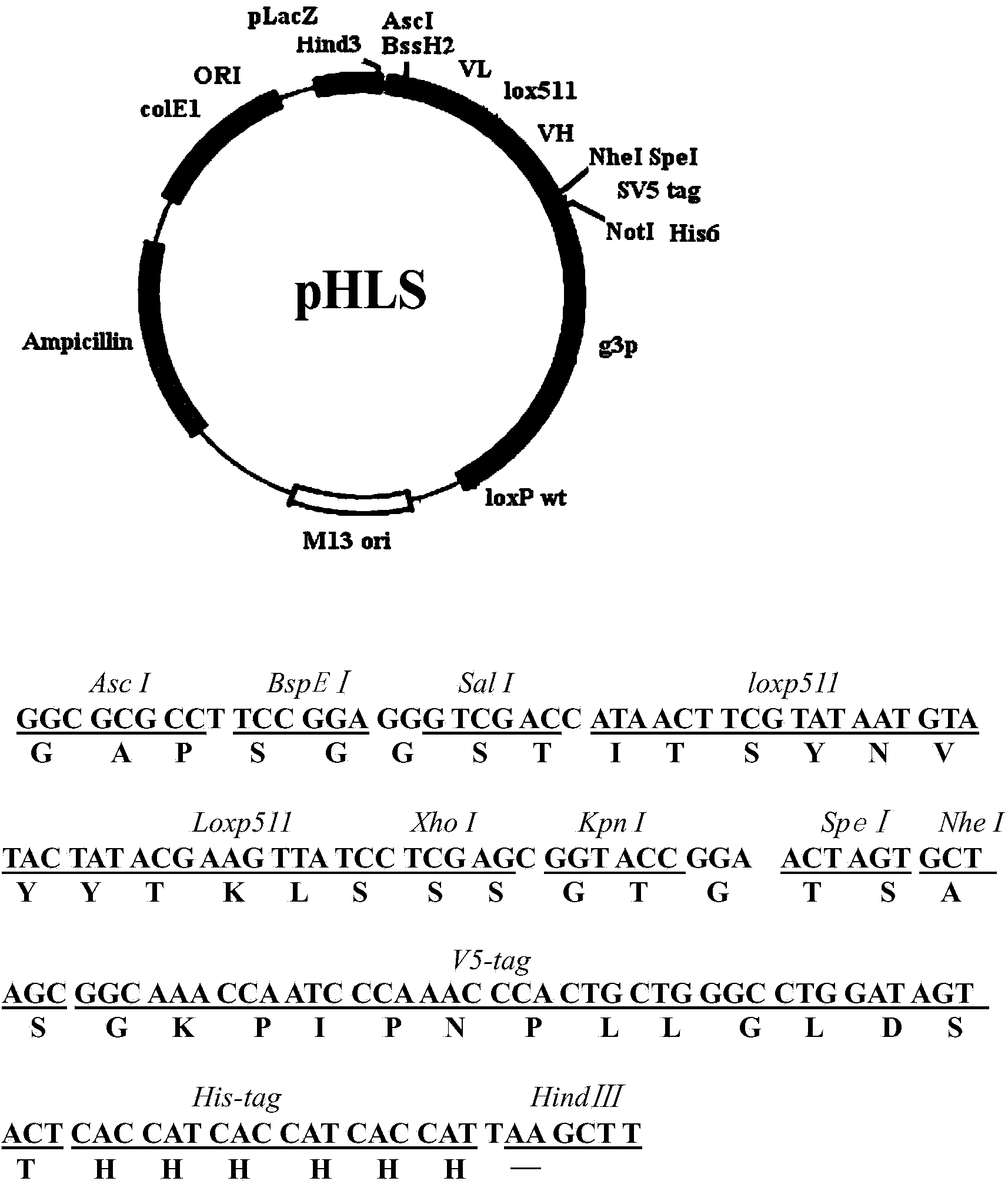
[0090] 1.1 Preparation of phagemid pHLS
[0091] 1) On the basis of the phagemid pUC119 vector (purchased from TAKARA company), design the gene sequence, such as figure 1 , using the whole gene synthesis technology to construct the phagemid pHLS.
[0092]2) Transfer the plasmid into XL1-blue (invitrogen) competent cells, pick a single colony, and insert 5mL2YT-A + In the culture solution (16g peptone, 10g yeast powder, 5g sodium chloride, add water to 1000mL, pH7.0, containing 100μg / mL ampicillin), transfer the next day to 200mL 2YT-A + culture solution overnight.
[0093] 3) Use the Qiagen plasmid extraction kit to extract the plasmid according to the kit instructions. Finally, the DNA concentration was detected in an ultraviolet spectrophotometer, and the final concentration was about 0.3 μg / mL.
[0094] 1.2 Extraction of total RNA from lymphocytes
[0095] 1) A total of 65 sam...
preparation example 2
[0178] Preparation Example 2 Screening of fully human anti-IL-17A single chain antibody
[0179] 2.1 Preparation of helper phage
[0180] 1) Pick a single XL1-BLUE strain and inoculate it into 40mL SB (containing 10ug / mL tetracycline), and culture overnight at 37°C with shaking.
[0181] 2) Dilute 1:500 into 10mL of the same SB medium the next day, and culture with shaking at 37°C for 1h.
[0182] 3) Pick a single M13K07 phage plaque, inoculate it into the above 10mL bacterial solution, and incubate with shaking at 37°C for 2h.
[0183] 4) Add it to the SB medium containing 10 μg / mL tetracycline and 70 μg / mL kanamycin to 500 mL, and culture overnight at 37°C with shaking.
[0184] 5) Cultivate until OD600 is about 1, centrifuge at 12000rpm at 4°C for 15min. The supernatant was taken, aseptically divided into test tubes, 50ml per tube, and stored at 4°C.
[0185] 2.2 Titration of phage virus species
[0186] 1) Prepare 6 LB culture plates without any resistance.
[0187] ...
preparation example 3
[0208] Preparation Example 3 Screening and Identification
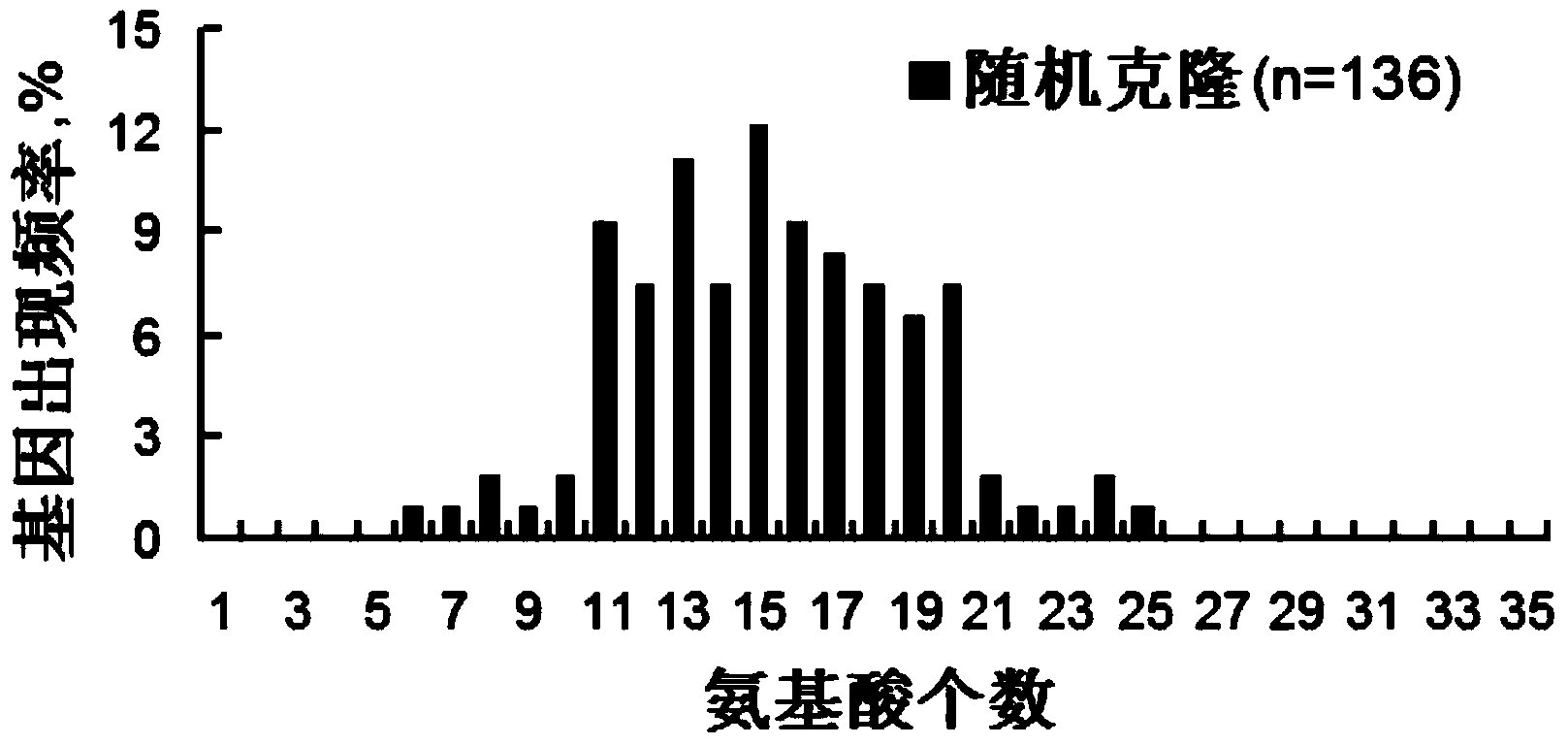
[0209] 3.1 Selection of random clones
[0210] 1) Prepare 2YT medium, along with 100 μg / mL ampicillin and 10 μg / mL tetracycline, and add it to a 96-well deep-well culture plate, about 600uL per empty.
[0211] 2) On the culture plate output from the third round and the fourth round of screening, a toothpick randomly selects colonies and inserts them into a 96-well deep-well culture plate. Incubate overnight at 37°C with shaking.
[0212] 3) The next day, transfer to a new 96-well deep-well culture plate containing 600uL medium at 1:10, and culture with shaking at 37°C for 3 hours. Add helper phage, incubate at 37°C for 20 minutes, and culture at 30°C for 8 hours with shaking.
[0213] 4) Centrifuge at 3000 rpm for 10 minutes. The supernatant was used as the scFv phage solution to be tested.
[0214] 3.2 Polyclonal phage ELISA
[0215] 1) Dilute human IL-17A recombinant protein and bovine serum albumin (BSA) to 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com