Stabilizing composition for biological materials
A composition, carbohydrate technology, applied in the direction of biochemical equipment and methods, microorganisms, microorganisms, etc., can solve problems such as protein and enzyme cell rupture
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] Preparation of dry and stable compositions
[0105] Basic Carb Mix
[0106] About 70 g of trehalose (Cargill Minneapolis, MN), about 5 g of instant inulin (Cargill Minneapolis, MN), and about 3 g of sodium alginate (ISP Corp., Wayne, NJ) were uniformly mixed in dry form.
[0107] Base Glass Reinforcing Mixture
[0108] About 17 g of casein hydrolyzate or pea hydrolyzate (ultrafiltered hydrolyzate, Marcor, Carlstadt, NJ) and 5 g of sodium citrate or sodium ascorbate (Sigma, St. Louis, MO) were uniformly mixed in dry form.
[0109] Stabilization of Probiotics
[0110] A fresh concentrate of L. rhamnosus (100ml, 10% solids, directly from the fermentation harvest) was added to the mixer and kept at 35°C. About 78 g of the base carbohydrate mix and about 22 g of the base glass enhancer mix were slowly added to the probiotic culture and mixed for 10 minutes at 35°C. The viscous slurry was then transferred to a container with a porous bottom and allowed to drip into a tank...
Embodiment 2
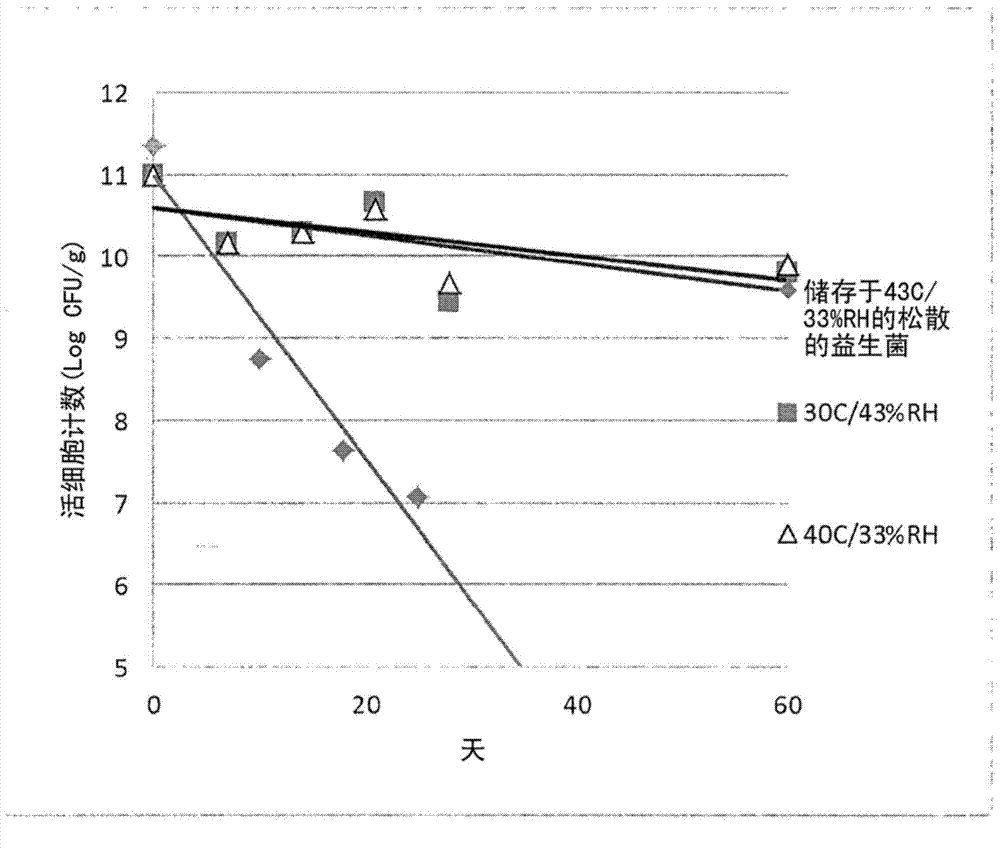
[0114] Storage Stability of Dried Probiotics
[0115] figure 1 The dry stable probiotics of Example 1 and commercially available dry probiotics (Culturelle, Amerifit, Inc., Cromwell, CT) were shown in two different accelerated storage conditions (40° C. and 33% RH and 30 Storage stability under ℃ and 43% RH). Commercially available probiotics completely lost their viability within the first few weeks under accelerated storage conditions, while the dry composition of the probiotics of the present invention lost only 1.18 log after 60 days at 30°C and 43% RH, at Only 1.09 log loss at 40°C and 33% RH.
Embodiment 3
[0117] Scaled-up production of a stable dry composition containing the probiotic Lactobacillus rhamnosus
[0118] Lactobacillus rhamnosus (400 g of frozen concentrate from a commercial source) was thawed at 37°C in a jacketed dual planetary mixer (DPM, 1qt, Ross Engineering, Inc. Savannah, GA) and the solids content was adjusted with distilled water. Adjust to 10% solids by weight. About 212 g of trehalose (Cargill Minneapolis, MN), about 20 g of instant inulin (Cargill Minneapolis, MN), about 12 g of sodium alginate (ISP Corp., Wayne, NJ), about 136 g of casein hydrolyzate ( Ultrafiltered hydrolyzate, Marcor, Carlstadt, NJ) and approximately 20 g of sodium ascorbate (Sigma, St. Louis, MO) were uniformly mixed in dry form. The powder mixture was slowly added to the probiotic culture and mixed for 10 minutes at 40 RPM, 37°C. The slurry was then transferred to a container with a porous bottom and allowed to drop into a tank containing liquid nitrogen. The beads were then remo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com